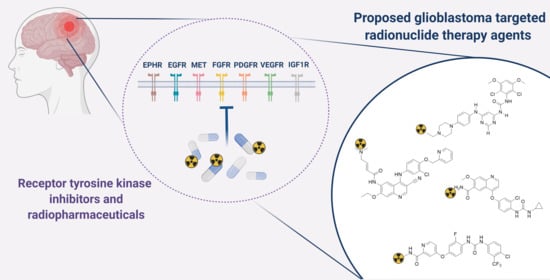
Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma
Abstract
:1. Introduction
2. Nuclear Molecular Imaging and TRT Using TKIs
3. Receptor Tyrosine Kinase Inhibitors (RTKIs) for GB Therapy
3.1. Epidermal Growth Factor Receptor (EGFR)
3.1.1. Current Status of EGFR and EGFRIII Targeted Therapy in GB
| Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference |
|---|---|---|---|
| Gefinitib (ZD1839) | SM | II (−) (after RT/chemo) | [88] |
| II (−) (RT) | [76] | ||
| I/II (−) (RT) | [89] | ||
| II (+/−) (cediranib) | [90] | ||
| Erlotinib (Tarceva, OSI-774) | SM | I (+) (RT) | [91] |
| II (−) | [92] | ||
| II (−) (RT + TMZ) | [93] | ||
| I/II (−) (single) | [94] | ||
| II (−) (single) | [95] | ||
| II (−) (TMZ/carmustine) | [78] | ||
| II (−) (sirolimus) | [77] | ||
| II (−) (RT/TMZ/bevacizumab) | [96] | ||
| II (−) (carboplatin) | [97] | ||
| Pilot (ongoing) (sunitinib, vandetanib) | NCT02239952 [67] | ||
| Lapatinib (GW572016) | SM | See Table 9 | |
| Afatinib (Tovok, BIBW2992) | SM | I/II (−) (TMZ) | [79] |
| I (ongoing) | NCT02423525 [67] | ||
| Dacomitinib (Vizimpro, PF299804) | SM | II (−) (single) | [98] |
| II (retrospective, subset +) | [80] | ||
| Vandetanib (Caprelsa, ZD6474) | SM | See Table 9 | |
| Tesevatinib (KD019/ XL647)> | SM | See Table 9 | |
| Osimertinib (AZD9291) | SM | II (recruiting) | NCT03732352 [67] |
| Everolimus (AEE788) | SM | See Table 9 | |
| Cetuximab (IMC-C225, Erbitux) | mAb | II (−) | [99] |
| II (−) (bevacizumab, irinotecan) | [100] | ||
| I/II (RT/TMZ) | [101] | ||
| II (ongoing) (RT) | NCT02800486 [67] | ||
| I/II (ongoing) (mannitol) | NCT02861898 [67] | ||
| Nimotuzumab (OSAG101) | mAb | II (+) (RT/chemo) | [102] |
| I/II (+) (RT) | [103] | ||
| I/II (+) (RT/chemo) | [104] | ||
| I/II (+/−) (RT/TMZ) | [105] | ||
| II/III (+) (RT) | [106,107] | ||
| III (+/−) (RT/chemo) | [108] | ||
| Panitumumab (Vectibix, ABX–EGF) | mAb | II (−) (irinotecan) | NCT01017653 [67] |
| GC1118 | mAb | II (ongoing) | NCT03618667 [67] |
| Depatuxizumab mafodotin (ABT-414/mAb 806) | Ab-drug | I (+/−) (single) | [109] |
| I (+) (TMZ) | [110] | ||
| I (+) (RT/TMZ) | [111] | ||
| I (+) (TMZ) | [112] | ||
| II (x) (TMZ/lomustine) | [113] | ||
| II/III (ongoing) (RT/TMZ) | NCT02573324 [67] | ||
| ABT 595 | Ab-drug | I (+) | [114] |
| Epitinib (HMPL-8) | SM | I (ongoing) | NCT03231501 [67] |
| Rindopepimut (CDX110) | Vaccine | II (+) (TMZ) | [73] |
| III (−) (TMZ) | [115] | ||
| II (+) (bevacizumab) | [116] | ||
| CART-EGFRvIII T | CARs | I (terminated) | [117] |
| I Pilot (−) | [118] | ||
| Anti-CD3/EGFR Bispecific Antibody Armed T Cells (EGFR BATs) | bAb-T | I (RT/TMZ) (ongoing) | NCT03344250 [67] |
| T Cells (EGFR BATs) | |||
| EGFR(V)-EDV-Dox | EDV | I (ongoing) | NCT02766699 [67] |
| AMG 596 | BiTE | I (single/AMG 404) (ongoing) | NCT03296696 [67] |
| Sym004 | Ab mix | II (completed, no results) | NCT02540161 [67] |
3.1.2. EGFR Radiopharmaceuticals
3.2. Vascular Endothelial Growth Factor Receptor (VEGFR)
3.2.1. Current Status of VEGFR Targeted Therapy in GB
| Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined or Compared Therapy) | Reference | |
|---|---|---|---|---|
| Bevacizumab | mAb | II (+) (single/irinotecan) | [198] | |
| II (+) (single) | [199] | |||
| II (+) (single) | [200] | |||
| II (+) (TMZ) | [201] | |||
| II (+) (TMZ) | [202] | |||
| II (+) (TMZ) | [203] | |||
| II (−) (TMZ) | [204] | |||
| II (−) (RT/hypoRT) | [205] | |||
| III (−) (RT/TMZ) | [181] | |||
| III (−) (RT/TMZ) | [180] | |||
| II (−) (RT/TMZ) | [206] | |||
| II (+) (RT/TMZ) | [207] | |||
| II (+) (RT/TMZ) | [208] | |||
| II (−) (hypoRT/TMZ) | [209] | |||
| II (−) (hypoRT/TMZ) | [210] | |||
| II (+) (irinotecan) | [211] | |||
| II (+) (irinotecan) | [212] | |||
| II (−) (irinotecan/TMZ) | [213] | |||
| II (+) (irinotecan/TMZ) | [214] | |||
| II (−) (irinotecan) | [215] | |||
| II (−) (irinotecan/TMZ) | [216] | |||
| II (−) (irinotecan/TMZ) | [217] | |||
| II (−) (cetuximab/irinotecan) | [100] | |||
| II (−) (TMZ/lomustine) | [218] | |||
| II (+) (lomustine) | [219] | |||
| II (−) (lomustine) | [220] | |||
| III (−) (lomustine) | [182] | |||
| II (−) (carboplatin) | [221] | |||
| II (−) (carboplatin/irinotecan) | [222] | |||
| II (+) (rindopepimut) | [116] | |||
| I/II (−) (BKM120) | [223] | |||
| I/II (−) (dasatinib) | [224] | |||
| II (+) (ERC1671 vaccine) | [225] | |||
| II (−) (onartuzumab) | [226] | |||
| II (−) (temsirolimus) | [227] | |||
| II (−) (tandutinib) | [228] | |||
| II (+) (fotemustine) | [229] | |||
| II (−) (fotemustine) | [230] | |||
| II (RT/TMZ/everolimus) | [231] | |||
| II (−) (metronomic etoposide/TMZ) | [232] | |||
| II (−) (panobinostat) | [233] | |||
| I (+) (DEHSRT#) | [234] | |||
| II (−) (sorafenib) | [235] | |||
| II (−) (erlotinib/RT/TMZ) | [96] | |||
| II (erlotinib) | [236] | |||
| II (−) (vorinostat) | [237] | |||
| I/II (−) (vorinostat/TMZ) | [238] | |||
| II (−) (enzastaurin) | [239] | |||
| Cediranib (AZD-2171) | SM | II (+) (single) | [189] | |
| III (−) (lomustine) | [190] | |||
| II (+) (gefinitib) | [90] | |||
| II (active, not recruiting) (olaparib) | NCT02974621 [67] | |||
| Aflibercept | Fusion protein * | II (−) | [184] | |
| I (+) (RT/TMZ) | [185] | |||
| Vatalinib (PTK787/ZK222584) | SM | I (+) (imatinib/hydroxyurea) | [188] | |
| I (+) II (term) (RT/TMZ) | [187] | |||
| I (+) (RT/TMZ/anti−epileptic drug) | [186] | |||
| Axitinib | SM | II (+) | [192] | |
| II (+) (lomustine) | [193] | |||
| II (−) (avelumab) | [191] | |||
| Tivozanib | SM | II (−) | [183] | |
| Ramucirumab | mAb | II (completed, no results) (IMC−3G3) | NCT00895180 [67] | |
| Sorafenib | SM |  | See Table 9 | |
| Sunitinib | SM | |||
| Nintedanib (BIBF 1120) | SM | |||
| Pazopanib (GW786034) | SM | |||
| Vandetanib (Caprelsa, ZD6474) | SM | |||
| Cabozantinib (XL-184) | SM | |||
| Regorafenib (BAY73-4506) | SM | |||
| Dovitinib (TKI258) | SM | |||
| Ponatinib (AP24534) | SM | |||
| Lenvatinib (E7080) | SM | |||
| Everolimus (AEE788) | SM | |||
| Anlotinib (AL3818) | SM | |||
3.2.2. VEGFR Radiopharmaceuticals
3.3. Mesenchymal-Epithelial Transition Factor (MET) Receptor
3.3.1. Current Status of MET Targeted Therapy in GB
| Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference |
|---|---|---|---|
| Onartuzumab | mAb | II (-) (bevacizumab) | [226] |
| Rilotumumab (AMG102) | mAb | II (−) | [261] |
| II (−) (bevacizumab) | [262] | ||
| Capmatinib (INC280) | SM | Ib/II (−) (buparlisib) | [265] |
| I (active, not recruiting) (bevacizumab) | NCT02386826 [67] | ||
| PLB-1001 (bozitinib) | SM | I (+) | [266] |
| Volitinib (savolitinib) | SM | I (recruiting) | NCT03598244 [67] |
| Crizotinib (PF-02341066) | SM | I (active, not recruiting) (RT/TMZ) | NCT02270034 [67] |
| Cabozantinib * (XL184) | SM | See Table 9 |
3.3.2. MET Radiopharmaceuticals
3.4. Platelet-Derived Growth Factor Receptor (PDGFR)
3.4.1. Current Status of PDGFR Targeted Therapy in GB
3.4.2. PDGFR Radiopharmaceuticals
3.5. Fibroblast Growth Factor Receptor (FGFR)
3.5.1. Current Status of FGFR Targeted Therapy in GB
3.5.2. FGFR Radiopharmaceuticals
3.6. Ehrin Receptors
3.6.1. Current Status of Eph Receptor Targeted Therapy in GB
| Target | Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference |
|---|---|---|---|---|
| Ephrin receptors | Tesevatinib (KD019/ XL647) | SM | See Table 9 | |
| Ifabotuzumab (KB004) | mAb | I (recruiting) (prelim) | [340] | |
| NCT0337494 [67] | ||||
| Dasatinib (BMS-354825) | SM | See Table 9 | ||
| IGF1R | Cixutumumab (IMC-A12) | mAb | I (withdrawn) (temsirolimus) | NCT01182883 [67] |
| IGF-1R/AS ODN * | as-odn | 0/I (+) | [348] | |
| 0/I (+) | [349] | |||
| PPP/AXL1717 | SM | I (+) | [350] | |
| I/II (unknown recruitment status) | NCT01721577 [67] |
3.6.2. Ehrin Receptor Radiopharmaceuticals
3.7. Insulin-Like Growth Factor 1 Receptor (IGF1R)
3.7.1. Current Status of IGF1R Targeted Therapy in GB
3.7.2. IGF1R Radiopharmaceuticals
4. Multi-Kinase Inhibition for GB Therapy
4.1. Current Status of Single Agent Multi-Kinase Inhibitors for GB Therapy
| Target | Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference |
|---|---|---|---|---|
| EGFR + HER2 | Lapatinib (Tykerb, GW572016) | SM | II (−) | [418] |
| I (+) | [420] | |||
| Pilot II (+) (RT/TMZ) | [419] | |||
| I/II (−) (pazopanib) | [421] | |||
| II (ongoing) (RT/TMZ) | NCT01591577 [67] | |||
| I (ongoing) (pre-surgery | NCT02101905 [67] | |||
| VEGFR-2 + EGFR + RET | Vandetanib (Caprelsa, ZD6474) | SM | I (+) (RT/TMZ) | [448] |
| I/II (−) | [405] | |||
| I (+) (Sirolimus) | [449] | |||
| II (−) (RT/TMZ) | [450] | |||
| Pilot (ongoing) (sunitinib, erlotinib) | NCT02239952 [67] | |||
| EGFR + HER1, HER2 and HER4 | Neratinib (Nerlynx™) | SM | II (TMZ) (recruiting) | [423] |
| c-MET, VEGFR-2, RET, KIT, FLT3, AXL and TEK | Cabozantinib (XL-184) | SM | I (+) (RT/TMZ) | [451] |
| II (modest) (received prior antiangiogenic therapy) | [401] | |||
| II (+/−) (naive to antiangiogenic therapy) | [403] | |||
| II (recruiting) | NCT02885324 [67] | |||
| VEGFR1–3 + TIE2 + KIT/RET/ RAF1/BRAF genes + PDGFR + FGFR + colony stimulating factor 1 receptor | Regorafenib (BAY73-4506) | SM | II (+) (vs. lomustine) | [408] |
| II (active, not recruiting) | NCT02926222 [67] | |||
| SRC + KIT + PDGFR + EPHA2 + BCR-ABL fusion | Dasatinib (BMS-354825) | SM | I/II (−) (CCNU) | [404] |
| II (−) | [402] | |||
| I (−) (bevacizumab) | [224] | |||
| I (+) (erlotinib) | [406] | |||
| PDGFRα/β + Bcr-Abl + c-FMS + c-Kit | Imatinib (Gleevec) | SM | II (+/−) (hydroxyurea) | [415] |
| I (+) (vatalinib/hydroxyurea) | [188] | |||
| I/II (−) (single) | [414] | |||
| II (−) | [412] | |||
| II (−) (RT/CCNU) | [413] | |||
| II (−) | [452] | |||
| VEGFR2/3 + Raf + PDGFR + c-KIT + Flt-3 | Sorafenib | SM | II (−) (RT/TMZ) | [428] |
| II (−) (TMZ) | [426] | |||
| II (+) (TMZ) | [453] | |||
| II (−) (erlotinib−EGFR) | [425] | |||
| II (−) (bevacizumab) | [235] | |||
| I (+) (RT/TMZ) | [454] | |||
| I/II (−) (temsirolimus) | [424] | |||
| I (−) (tipifarnib) | [429] | |||
| I (−) (RT/TMZ) | [427] | |||
| I/II (active NR) (everolimus) | NCT01434602 [67] | |||
| VEGFR1-2 + PDGFRβ + FGFR1-2-3 | Dovitinib (TKI258) | SM | I (+) | [455] |
| II (−) (no/prior bevacizumab) | [456] | |||
| PDGFR + VEGFR + FLT3 + RET | Sunitinib | SM | I (−) (irinotecan) | [432] |
| II (−) | [433] | |||
| II (−) (prior bevacizumab) | [430] | |||
| II (−) (RT) | [431] | |||
| II (−) | [434] | |||
| VEGFR1/2/3 + PDGFRα/β + c-Kit | Pazopanib (GW786034) | SM | I/II (−) (lapatinib) | [421] |
| II (−) (single) | [457] | |||
| PDGFR + VEGFR + Src + FGFR | Ponatinib (AP24534) | SM | II (−) (prior bevacizumab) | [438] |
| PDGFR α/β + FGFR 1-3 + VEGFR 1-3 | Nintedanib (BIBF 1120) | SM | II (−) (single) | [435] |
| II (−) (prior bevacizumab) | [436] | |||
| FGFR1-4, PDGFRβ, VEGFR1-3, RET, and KIT | Lenvatinib (E7080) | SM | II (modest) (prior bevacizumab) | [437] |
| EGFR + VEGF | Everolimus (AEE788) | SM | IB/II (−) (RAD001) | [85] |
| I (−) | [87] | |||
| VEGFR1/2/3 + FGFR1/2/3 + c-Kit + Ret | Anlotinib (AL3818) | SM | Case report (+) | [440] |
| Case report (+) | [439] | |||
| I/II (recruiting) | NCT04004975 [67] | |||
| II (recruiting) (TMZ) | NCT04547855 [67] | |||
| I/II (recruiting) (RT/TMZ) | NCT04157478 [67] | |||
| EGFR + VEGFR + EphB4 | Tesevatinib (KD019/XL647) | SM | II (completed, no results) | NCT02844439 [67] |
4.2. Current Status of Combined RTKI Therapy for GB
4.3. Multi-Kinase Targeted Radiopharmaceuticals
5. Selection and Radiolabeling of New TKIs for TRT of GB
6. Conclusive Statements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearson, J.R.D.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal. Transduct. Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. In Current Understanding and Treatment of Gliomas; Raizer, J., Parsa, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 163, pp. 1–14. [Google Scholar]
- Weller, M.; van den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma-are we there yet? Neuro Oncol. 2013, 15, 4–27. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Huse, J.T.; Holland, E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331. [Google Scholar] [CrossRef]
- Caragher, S.; Miska, J.; Shireman, J.; Park, C.H.; Muroski, M.; Lesniak, M.S.; Ahmed, A.U. Temozolomide Treatment Increases Fatty Acid Uptake in Glioblastoma Stem Cells. Cancers 2020, 12, 3126. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Theek, B.; Storm, G.; Kiessling, F.; Lammers, T. Recent progress in nanomedicine: Therapeutic, diagnostic and theranostic applications. Curr. Opin. Biotechnol. 2013, 24, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Puttemans, J.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; Vidal, A.; Bonnemaire, C.; Kraeber-Bodéré, F.; Chérel, M.; Pallardy, A.; Rousseau, C.; Garcion, E.; Lacoeuille, F.; Hindré, F.; et al. Potential for Nuclear Medicine Therapy for Glioblastoma Treatment. Front. Pharmacol. 2019, 10, 772. [Google Scholar] [CrossRef]
- Pruis, I.J.; van Dongen, G.; Veldhuijzen van Zanten, S.E.M. The Added Value of Diagnostic and Theranostic PET Imaging for the Treatment of CNS Tumors. Int. J. Mol. Sci. 2020, 21, 1029. [Google Scholar] [CrossRef] [Green Version]
- Bolcaen, J.; Kleynhans, J.; Nair, S.; Verhoeven, J.; Goethals, I.; Sathekge, M.; Vandevoorde, C.; Ebenhan, T. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics 2021. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [Green Version]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18, 2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slobbe, P.; Poot, A.J.; Windhorst, A.D.; van Dongen, G.A. PET imaging with small-molecule tyrosine kinase inhibitors: TKI-PET. Drug Discov. Today 2012, 17, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, O.; Purcaru, S.O.; Tataranu, L.G.; Lucan, L.; Castro, J.; Folcuţi, C.; Artene, S.A.; Tuţă, C.; Dricu, A. The Influence of EGFR Inactivation on the Radiation Response in High Grade Glioma. Int. J. Mol. Sci 2018, 19, 229. [Google Scholar] [CrossRef] [Green Version]
- Hintelmann, K.; Kriegs, M.; Rothkamm, K.; Rieckmann, T. Improving the Efficacy of Tumor Radiosensitization Through Combined Molecular Targeting. Front. Oncol. 2020, 10, 1260. [Google Scholar] [CrossRef]
- Wei, W.; Ni, D.; Ehlerding, E.B.; Luo, Q.Y.; Cai, W. PET Imaging of Receptor Tyrosine Kinases in Cancer. Mol. Cancer Ther. 2018, 17, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- Bernard-Gauthier, V.; Bailey, J.J.; Berke, S.; Schirrmacher, R. Recent Advances in the Development and Application of Radiolabeled Kinase Inhibitors for PET Imaging. Molecules 2015, 20, 22000–22027. [Google Scholar] [CrossRef] [Green Version]
- St James, S.; Bednarz, B.; Benedict, S.; Buchsbaum, J.C.; Dewaraja, Y.; Frey, E.; Hobbs, R.; Grudzinski, J.; Roncali, E.; Sgouros, G.; et al. Current Status of Radiopharmaceutical Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 891–901. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Cardinal Health. Available online: https://www.cardinalhealth.com/content/dam/corp/web/documents/fact-sheet/cardinal-health-fda-approved-radiopharmaceuticals.pdf (accessed on 22 May 2021).
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic Radiometals: Worldwide Scientific Literature Trend Analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef] [Green Version]
- Oriuchi, N.; Higuchi, T.; Hanaoka, H.; Iida, Y.; Endo, K. Current status of cancer therapy with radiolabeled monoclonal antibody. Ann. Nucl. Med. 2005, 19, 355–365. [Google Scholar] [CrossRef]
- Sugiura, G.; Kühn, H.; Sauter, M.; Haberkorn, U.; Mier, W. Radiolabeling strategies for tumor-targeting proteinaceous drugs. Molecules 2014, 19, 2135–2165. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Affleck, D.J.; Li, J.; Welsh, P.; Zalutsky, M.R. A polar substituent-containing acylation agent for the radioiodination of internalizing monoclonal antibodies: N-succinimidyl 4-guanidinomethyl-3-[131I]iodobenzoate ([131I]SGMIB). Bioconjug. Chem. 2001, 12, 428–438. [Google Scholar] [CrossRef]
- Chopra, A. [(125)I]-Labeled monoclonal antibody L8A4 against epidermal growth factor receptor variant III (EGFRvIII). In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Morais, M.; Ma, M.T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef]
- Okoye, N.C.; Baumeister, J.E.; Najafi, K.F.; Hennkens, H.M.; Jurisson, S.S. Chelator and metal complex stability for radiopharmaceutical applications. Radiochim. Acta 2019, 107, 1087–1120. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Sarko, D.; Eisenhut, M.; Haberkorn, U.; Mier, W. Bifunctional chelators in the design and application of radiopharmaceuticals for oncological diseases. Curr. Med. Chem. 2012, 19, 2667–2688. [Google Scholar] [CrossRef]
- Wängler, B.; Schirrmacher, R.; Bartenstein, P.; Wängler, C. Chelating agents and their use in radiopharmaceutical sciences. Mini Rev. Med. Chem. 2011, 11, 968–983. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, V.; Orlova, A. Influence of labelling methods on biodistribution and imaging properties of radiolabelled peptides for visualisation of molecular therapeutic targets. Curr. Med. Chem. 2010, 17, 2636–2655. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Stigter-van Walsum, M.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar] [PubMed]
- Chen, W.; Shen, B.; Sun, X. Analysis of Progress and Challenges of EGFR-Targeted Molecular Imaging in Cancer with a Focus on Affibody Molecules. Mol. Imaging 2019, 18, 1536012118823473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dongen, G.A.; Poot, A.J.; Vugts, D.J. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: Immuno-PET and TKI-PET. Tumour Biol. 2012, 33, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolmachev, V.; Stone-Elander, S.; Orlova, A. Radiolabelled receptor-tyrosine-kinase targeting drugs for patient stratification and monitoring of therapy response: Prospects and pitfalls. Lancet Oncol. 2010, 11, 992–1000. [Google Scholar] [CrossRef]
- Hicks, J.W.; VanBrocklin, H.F.; Wilson, A.A.; Houle, S.; Vasdev, N. Radiolabeled small molecule protein kinase inhibitors for imaging with PET or SPECT. Molecules 2010, 15, 8260–8278. [Google Scholar] [CrossRef] [Green Version]
- Altai, M.; Orlova, A.; Tolmachev, V. Radiolabeled probes targeting tyrosine-kinase receptors for personalized medicine. Curr. Pharm. Des. 2014, 20, 2275–2292. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Moreau, M.; Raguin, O.; Oudot, A.; Bernhard, C.; Vrigneaud, J.M.; Dumont, L.; Vandroux, D.; Denat, F.; Cochet, A.; et al. Radiolabeled F(ab′)(2)-cetuximab for theranostic purposes in colorectal and skin tumor-bearing mice models. Clin. Transl. Oncol. 2018, 20, 1557–1570. [Google Scholar] [CrossRef] [Green Version]
- Casacó, A.; López, G.; García, I.; Rodríguez, J.A.; Fernández, R.; Figueredo, J.; Torres, L.; Perera, A.; Batista, J.; Leyva, R.; et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol. Ther. 2008, 7, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Emrich, J.G.; Brady, L.W.; Quang, T.S.; Class, R.; Miyamoto, C.; Black, P.; Rodeck, U. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: Ten-year synopsis of a novel treatment. Am. J. Clin. Oncol. 2002, 25, 541–546. [Google Scholar] [CrossRef]
- Hens, M.; Vaidyanathan, G.; Zhao, X.G.; Bigner, D.D.; Zalutsky, M.R. Anti-EGFRvIII monoclonal antibody armed with 177Lu: In Vivo comparison of macrocyclic and acyclic ligands. Nucl. Med. Biol. 2010, 37, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Hens, M.; Vaidyanathan, G.; Welsh, P.; Zalutsky, M.R. Labeling internalizing anti-epidermal growth factor receptor variant III monoclonal antibody with (177)Lu: In Vitro comparison of acyclic and macrocyclic ligands. Nucl. Med. Biol. 2009, 36, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Reist, C.J.; Foulon, C.F.; Alston, K.; Bigner, D.D.; Zalutsky, M.R. Astatine-211 labeling of internalizing anti-EGFRvIII monoclonal antibody using N-succinimidyl 5-[211At]astato-3-pyridinecarboxylate. Nucl. Med. Biol. 1999, 26, 405–411. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Shetake, N.G.; Pandey, B.N.; Kumar, A. Receptor tyrosine kinase signaling in cancer radiotherapy and its targeting for tumor radiosensitization. Int. J. Radiat. Biol. 2018, 94, 628–644. [Google Scholar] [CrossRef]
- Carrasco-García, E.; Saceda, M.; Martínez-Lacaci, I. Role of receptor tyrosine kinases and their ligands in glioblastoma. Cells 2014, 3, 199–235. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Puputti, M.; Sihto, H.; Tynninen, O.; Nupponen, N.N. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J. Pathol. 2005, 207, 224–231. [Google Scholar] [CrossRef]
- Day, B.W.; Stringer, B.W.; Boyd, A.W. Eph receptors as therapeutic targets in glioblastoma. Br. J. Cancer 2014, 111, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Ko, Y.T. Small molecule tyrosine kinase inhibitors in glioblastoma. Arch. Pharm. Res. 2020, 43, 385–394. [Google Scholar] [CrossRef]
- Maris, C.; D’Haene, N.; Trépant, A.L.; Le Mercier, M.; Sauvage, S.; Allard, J.; Rorive, S.; Demetter, P.; Decaestecker, C.; Salmon, I. IGF-IR: A new prognostic biomarker for human glioblastoma. Br. J. Cancer 2015, 113, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [Green Version]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 15 January 2021).
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Furnari, F.B.; Cloughesy, T.F.; Cavenee, W.K.; Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 2015, 15, 302–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oprita, A.; Baloi, S.C.; Staicu, G.A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Maire, C.L.; Lamszus, K. EGFR as a Target for Glioblastoma Treatment: An Unfulfilled Promise. CNS Drugs 2017, 31, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Eskilsson, E.; Røsland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol. 2018, 20, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol. 2015, 17, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Harari, P.M. Epidermal growth factor receptor inhibition strategies in oncology. Endocr. Relat. Cancer 2004, 11, 689–708. [Google Scholar] [CrossRef] [Green Version]
- Uhm, J.H.; Ballman, K.V.; Wu, W.; Giannini, C.; Krauss, J.C.; Buckner, J.C.; James, C.D.; Scheithauer, B.W.; Behrens, R.J.; Flynn, P.J.; et al. Phase II evaluation of gefitinib in patients with newly diagnosed Grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E., 2nd; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 2010, 96, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Van den Bent, M.J.; Brandes, A.A.; Rampling, R.; Kouwenhoven, M.C.; Kros, J.M.; Carpentier, A.F.; Clement, P.M.; Frenay, M.; Campone, M.; Baurain, J.F.; et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009, 27, 1268–1274. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Nabors, L.B.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro Oncol. 2015, 17, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Chi, A.S.; Cahill, D.P.; Reardon, D.A.; Wen, P.Y.; Mikkelsen, T.; Peereboom, D.M.; Wong, E.T.; Gerstner, E.R.; Dietrich, J.; Plotkin, S.R.; et al. Exploring Predictors of Response to Dacomitinib in EGFR-Amplified Recurrent Glioblastoma. JCO Precis. Oncol. 2020, 4. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Nimmakayala, R.K.; Rachagani, S.; Siddiqui, J.A.; Mallya, K.; Gorantla, S.; Jain, M.; Ponnusamy, M.P.; Batra, S.K.; et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 266. [Google Scholar] [CrossRef]
- Chagoya, G.; Kwatra, S.G.; Nanni, C.W.; Roberts, C.M.; Phillips, S.M.; Nullmeyergh, S.; Gilmore, S.P.; Spasojevic, I.; Corcoran, D.L.; Young, C.C.; et al. Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 2020, 11, 2074–2082. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Shi, L.; Shan, Q.; Cao, Q.; Yue, C.; Li, H.; Li, S.; Wang, J.; Gao, S.; et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 219. [Google Scholar] [CrossRef]
- Makhlin, I.; Salinas, R.D.; Zhang, D.; Jacob, F.; Ming, G.L.; Song, H.; Saxena, D.; Dorsey, J.F.; Nasrallah, M.P.; Morrissette, J.J.; et al. Clinical activity of the EGFR tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol. 2019, 8, Cns43. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Cloughesy, T.; Rich, J.; Alfred Yung, W.K.; Yung, L.; DiLea, C.; Huang, J.; Dugan, M.; Mietlowski, W.; Maes, A.; et al. Pharmacokinetic drug interaction between AEE788 and RAD001 causing thrombocytopenia in patients with glioblastoma. Cancer Chemother. Pharmacol. 2012, 69, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Goudar, R.K.; Shi, Q.; Hjelmeland, M.D.; Keir, S.T.; McLendon, R.E.; Wikstrand, C.J.; Reese, E.D.; Conrad, C.A.; Traxler, P.; Lane, H.A.; et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol. Cancer Ther. 2005, 4, 101–112. [Google Scholar] [PubMed]
- Reardon, D.A.; Conrad, C.A.; Cloughesy, T.; Prados, M.D.; Friedman, H.S.; Aldape, K.D.; Mischel, P.; Xia, J.; DiLea, C.; Huang, J.; et al. Phase I study of AEE788, a novel multitarget inhibitor of ErbB- and VEGF-receptor-family tyrosine kinases, in recurrent glioblastoma patients. Cancer Chemother. Pharmacol. 2012, 69, 1507–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, E.; Cavallo, G.; Lonardi, S.; Magrini, E.; Tosoni, A.; Grosso, D.; Scopece, L.; Blatt, V.; Urbini, B.; Pession, A.; et al. Gefitinib in patients with progressive high-grade gliomas: A multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br. J. Cancer 2007, 96, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Wang, M.; Robins, H.I.; Lautenschlaeger, T.; Curran, W.J.; Brachman, D.G.; Schultz, C.J.; Choucair, A.; Dolled-Filhart, M.; Christiansen, J.; et al. RTOG 0211: A phase 1/2 study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1206–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K.; et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE 2016, 11, e0156369. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Brown, P.D.; Ballman, K.V.; Fiveash, J.B.; Uhm, J.H.; Giannini, C.; Jaeckle, K.A.; Geoffroy, F.J.; Nabors, L.B.; Buckner, J.C. Phase I trial of erlotinib with radiation therapy in patients with glioblastoma multiforme: Results of North Central Cancer Treatment Group protocol N0177. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1192–1199. [Google Scholar] [CrossRef]
- Yung, W.K.; Vredenburgh, J.J.; Cloughesy, T.F.; Nghiemphu, P.; Klencke, B.; Gilbert, M.R.; Reardon, D.A.; Prados, M.D. Safety and efficacy of erlotinib in first-relapse glioblastoma: A phase II open-label study. Neuro Oncol. 2010, 12, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Peereboom, D.M.; Shepard, D.R.; Ahluwalia, M.S.; Brewer, C.J.; Agarwal, N.; Stevens, G.H.; Suh, J.H.; Toms, S.A.; Vogelbaum, M.A.; Weil, R.J.; et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 2010, 98, 93–99. [Google Scholar] [CrossRef]
- Kesavabhotla, K.; Schlaff, C.D.; Shin, B.; Mubita, L.; Kaplan, R.; Tsiouris, A.J.; Pannullo, S.C.; Christos, P.; Lavi, E.; Scheff, R.; et al. Phase I/II study of oral erlotinib for treatment of relapsed/refractory glioblastoma multiforme and anaplastic astrocytoma. J. Exp. Ther. Oncol. 2012, 10, 71–81. [Google Scholar]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.; Gilbert, M.R.; Aldape, K.A.; Wen, P.Y.; et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010, 12, 95–103. [Google Scholar] [CrossRef]
- Clarke, J.L.; Molinaro, A.M.; Phillips, J.J.; Butowski, N.A.; Chang, S.M.; Perry, A.; Costello, J.F.; DeSilva, A.A.; Rabbitt, J.E.; Prados, M.D. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol. 2014, 16, 984–990. [Google Scholar] [CrossRef] [Green Version]
- De Groot, J.F.; Gilbert, M.R.; Aldape, K.; Hess, K.R.; Hanna, T.A.; Ictech, S.; Groves, M.D.; Conrad, C.; Colman, H.; Puduvalli, V.K.; et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J. Neurooncol. 2008, 90, 89–97. [Google Scholar] [CrossRef]
- Sepúlveda-Sánchez, J.M.; Vaz, M.; Balañá, C.; Gil-Gil, M.; Reynés, G.; Gallego, Ó.; Martínez-García, M.; Vicente, E.; Quindós, M.; Luque, R.; et al. Phase II trial of dacomitinib, a pan-human EGFR tyrosine kinase inhibitor, in recurrent glioblastoma patients with EGFR amplification. Neuro Oncol. 2017, 19, 1522–1531. [Google Scholar] [CrossRef] [Green Version]
- Neyns, B.; Sadones, J.; Joosens, E.; Bouttens, F.; Verbeke, L.; Baurain, J.F.; D’Hondt, L.; Strauven, T.; Chaskis, C.; In’t Veld, P.; et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann. Oncol. 2009, 20, 1596–1603. [Google Scholar] [CrossRef]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sørensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.T.; Poulsen, H.S. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro Oncol. 2010, 12, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Combs, S.E.; Heeger, S.; Haselmann, R.; Edler, L.; Debus, J.; Schulz-Ertner, D. Treatment of primary glioblastoma multiforme with cetuximab, radiotherapy and temozolomide (GERT)--phase I/II trial: Study protocol. BMC Cancer 2006, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Du, X.J.; Li, X.M.; Cai, L.B.; Sun, J.C.; Wang, S.Y.; Wang, X.C.; Pang, X.L.; Deng, M.L.; Chen, F.F.; Wang, Z.Q.; et al. Efficacy and safety of nimotuzumab in addition to radiotherapy and temozolomide for cerebral glioblastoma: A phase II multicenter clinical trial. J. Cancer 2019, 10, 3214–3223. [Google Scholar] [CrossRef] [Green Version]
- Ramos, T.C.; Figueredo, J.; Catala, M.; González, S.; Selva, J.C.; Cruz, T.M.; Toledo, C.; Silva, S.; Pestano, Y.; Ramos, M.; et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: Report from a phase I/II trial. Cancer Biol. Ther. 2006, 5, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Peng, Y.; Liao, Y.; Jiang, W.; Wei, R.; Huo, L.; Han, Z.; Duan, C.; Zhong, M. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp. Ther. Med. 2012, 4, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pan, L.; Sheng, X.F.; Chen, S.; Dai, J.Z. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac. J. Clin. Oncol. 2016, 12, e23–e29. [Google Scholar] [CrossRef]
- Solomon, M.T.; Miranda, N.; Jorrín, E.; Chon, I.; Marinello, J.J.; Alert, J.; Lorenzo-Luaces, P.; Crombet, T. Nimotuzumab in combination with radiotherapy in high grade glioma patients: A single institution experience. Cancer Biol. Ther. 2014, 15, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Solomón, M.T.; Selva, J.C.; Figueredo, J.; Vaquer, J.; Toledo, C.; Quintanal, N.; Salva, S.; Domíngez, R.; Alert, J.; Marinello, J.J.; et al. Radiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: Results from a randomized, double blind trial. BMC Cancer 2013, 13, 299. [Google Scholar] [CrossRef] [Green Version]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef]
- Van den Bent, M.; Gan, H.K.; Lassman, A.B.; Kumthekar, P.; Merrell, R.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: Results from a multi-center, international study. Cancer Chemother. Pharmacol. 2017, 80, 1209–1217. [Google Scholar] [CrossRef]
- Lassman, A.B.; van den Bent, M.J.; Gan, H.K.; Reardon, D.A.; Kumthekar, P.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P.; et al. Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: Results from an international phase I multicenter trial. Neuro Oncol. 2019, 21, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Lassman, A.B.; van den Bent, M.; Kumthekar, P.; Merrell, R.; Scott, A.M.; Fichtel, L.; Sulman, E.P.; Gomez, E.; Fischer, J.; et al. Efficacy and safety results of ABT-414 in combination with radiation and temozolomide in newly diagnosed glioblastoma. Neuro Oncol. 2017, 19, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Gan, H.K.; Reardon, D.A.; Lassman, A.B.; Merrell, R.; van den Bent, M.; Butowski, N.; Lwin, Z.; Wheeler, H.; Fichtel, L.; Scott, A.M.; et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2018, 20, 838–847. [Google Scholar] [CrossRef]
- Van Den Bent, M.; Eoli, M.; Sepulveda, J.M.; Smits, M.; Walenkamp, A.; Frenel, J.S.; Franceschi, E.; Clement, P.M.; Chinot, O.; De Vos, F.; et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020, 22, 684–693. [Google Scholar] [CrossRef]
- Rosenthal, M.; Curry, R.; Reardon, D.A.; Rasmussen, E.; Upreti, V.V.; Damore, M.A.; Henary, H.A.; Hill, J.S.; Cloughesy, T. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody-drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother. Pharmacol. 2019, 84, 327–336. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V.; et al. Rindopepimut with Bevacizumab for Patients with Relapsed EGFRvIII-Expressing Glioblastoma (ReACT): Results of a Double-Blind Randomized Phase II Trial. Clin. Cancer Res. 2020, 26, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Gan, H.K.; Burgess, A.W.; Clayton, A.H.; Scott, A.M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012, 72, 2924–2930. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Kozlosky, C.J.; Siu, S.; Chang, W.S.; Liu, H.; Foltz, I.N.; Trueblood, E.S.; Meininger, D.; Arora, T.; Twomey, B.; et al. AMG 595, an Anti-EGFRvIII Antibody-Drug Conjugate, Induces Potent Antitumor Activity against EGFRvIII-Expressing Glioblastoma. Mol. Cancer Ther. 2015, 14, 1614–1624. [Google Scholar] [CrossRef] [Green Version]
- Struve, N.; Binder, Z.A.; Stead, L.F.; Brend, T.; Bagley, S.J.; Faulkner, C.; Ott, L.; Müller-Goebel, J.; Weik, A.S.; Hoffer, K.; et al. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene 2020, 39, 3041–3055. [Google Scholar] [CrossRef] [Green Version]
- Pan, P.C.; Magge, R.S. Mechanisms of EGFR Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 8471. [Google Scholar] [CrossRef]
- Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [Google Scholar] [CrossRef]
- Ronellenfitsch, M.W.; Zeiner, P.S.; Mittelbronn, M.; Urban, H.; Pietsch, T.; Reuter, D.; Senft, C.; Steinbach, J.P.; Westphal, M.; Harter, P.N. Akt and mTORC1 signaling as predictive biomarkers for the EGFR antibody nimotuzumab in glioblastoma. Acta Neuropathol. Commun. 2018, 6, 81. [Google Scholar] [CrossRef]
- Muñoz-Hidalgo, L.; San-Miguel, T.; Megías, J.; Monleón, D.; Navarro, L.; Roldán, P.; Cerdá-Nicolás, M.; López-Ginés, C. Somatic copy number alterations are associated with EGFR amplification and shortened survival in patients with primary glioblastoma. Neoplasia 2020, 22, 10–21. [Google Scholar] [CrossRef]
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Vicente, M. Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Farag, A.K.; Viswanath, A.N.; Bedair, T.M.; Leem, D.G.; Lee, K.T.; Pae, A.N.; Roh, E.J. Targeting EGFR/HER2 tyrosine kinases with a new potent series of 6-substituted 4-anilinoquinazoline hybrids: Design, synthesis, kinase assay, cell-based assay, and molecular docking. Bioorg. Med. Chem. Lett. 2015, 25, 5147–5154. [Google Scholar] [CrossRef]
- Li, L.; Quang, T.S.; Gracely, E.J.; Kim, J.H.; Emrich, J.G.; Yaeger, T.E.; Jenrette, J.M.; Cohen, S.C.; Black, P.; Brady, L.W. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010, 113, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Cai, L.; Zhang, K.; Zhang, A.; Pu, P.; Yang, W.; Gao, S. A pilot study on EGFR-targeted molecular imaging of PET/CT with 11C-PD153035 in human gliomas. Clin. Nucl. Med. 2014, 39, e20–e26. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Li, X.; Meng, X.; Yang, G.; Zhao, S.; Yang, Y.; Ma, L.; Fu, Z.; Yu, J. PET-based biodistribution and radiation dosimetry of epidermal growth factor receptor-selective tracer 11C-PD153035 in humans. J. Nucl. Med. 2009, 50, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Petrulli, J.R.; Sullivan, J.M.; Zheng, M.Q.; Bennett, D.C.; Charest, J.; Huang, Y.; Morris, E.D.; Contessa, J.N. Quantitative analysis of [11C]-erlotinib PET demonstrates specific binding for activating mutations of the EGFR kinase domain. Neoplasia 2013, 15, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Traxl, A.; Mairinger, S.; Filip, T.; Sauberer, M.; Stanek, J.; Poschner, S.; Jäger, W.; Zoufal, V.; Novarino, G.; Tournier, N.; et al. Inhibition of ABCB1 and ABCG2 at the Mouse Blood-Brain Barrier with Marketed Drugs to Improve Brain Delivery of the Model ABCB1/ABCG2 Substrate [(11)C]erlotinib. Mol. Pharm. 2019, 16, 1282–1293. [Google Scholar] [CrossRef]
- Tournier, N.; Goutal, S.; Mairinger, S.; Hernández-Lozano, I.; Filip, T.; Sauberer, M.; Caillé, F.; Breuil, L.; Stanek, J.; Freeman, A.F.; et al. Complete inhibition of ABCB1 and ABCG2 at the blood-brain barrier by co-infusion of erlotinib and tariquidar to improve brain delivery of the model ABCB1/ABCG2 substrate [(11)C]erlotinib. J. Cereb. Blood Flow Metab. 2020, 271678x20965500. [Google Scholar] [CrossRef]
- Shamni, O.; Grievink, H.; Itamar, B.; Mishani, E.; Abourbeh, G. Development of a Fluorinated Analogue of Erlotinib for PET Imaging of EGFR Mutation-Positive NSCLC. Mol. Imaging Biol. 2019, 21, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Seimbille, Y.; Phelps, M.E.; Czernin, J.; Silverman, D.H.S. Fluorine-18 labeling of 6,7-disubstituted anilinoquinazoline derivatives for positron emission tomography (PET) imaging of tyrosine kinase receptors: Synthesis of 18F-Iressa and related molecular probes. J. Label. Compd. Radiopharm. 2005, 48, 829–843. [Google Scholar] [CrossRef]
- Huang, S.; Han, Y.; Chen, M.; Hu, K.; Qi, Y.; Sun, P.; Wang, M.; Wu, H.; Li, G.; Wang, Q.; et al. Radiosynthesis and biological evaluation of 18F-labeled 4-anilinoquinazoline derivative (18F-FEA-Erlotinib) as a potential EGFR PET agent. Bioorg. Med. Chem. Lett. 2018, 28, 1143–1148. [Google Scholar] [CrossRef]
- Vlaming, M.L.; Läppchen, T.; Jansen, H.T.; Kivits, S.; van Driel, A.; van de Steeg, E.; van der Hoorn, J.W.; Sio, C.F.; Steinbach, O.C.; DeGroot, J. PET-CT imaging with [18F]-gefitinib to measure Abcb1a/1b (P-gp) and Abcg2 (Bcrp1) mediated drug-drug interactions at the murine blood-brain barrier. Nucl. Med. Biol. 2015, 42, 833–841. [Google Scholar] [CrossRef]
- Su, H.; Seimbille, Y.; Ferl, G.Z.; Bodenstein, C.; Fueger, B.; Kim, K.J.; Hsu, Y.T.; Dubinett, S.M.; Phelps, M.E.; Czernin, J.; et al. Evaluation of [18F]gefitinib as a molecular imaging probe for the assessment of the epidermal growth factor receptor status in malignant tumors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1089–1099. [Google Scholar] [CrossRef]
- Wang, J.Q.; Gao, M.; Miller, K.D.; Sledge, G.W.; Zheng, Q.H. Synthesis of [11C]Iressa as a new potential PET cancer imaging agent for epidermal growth factor receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2006, 16, 4102–4106. [Google Scholar] [CrossRef]
- Holt, D.P.; Ravert, H.T.; Dannals, R.F.; Pomper, M.G. Synthesis of [11C]gefitinib for imaging epidermal growth factor receptor tyrosine kinase with positron emission tomography. J. Label. Compd. Radiopharm. 2006, 49, 883–888. [Google Scholar] [CrossRef]
- Bonasera, T.A.; Ortu, G.; Rozen, Y.; Krais, R.; Freedman, N.M.; Chisin, R.; Gazit, A.; Levitzki, A.; Mishani, E. Potential (18)F-labeled biomarkers for epidermal growth factor receptor tyrosine kinase. Nucl. Med. Biol. 2001, 28, 359–374. [Google Scholar] [CrossRef]
- Ortu, G.; Ben-David, I.; Rozen, Y.; Freedman, N.M.; Chisin, R.; Levitzki, A.; Mishani, E. Labeled EGFr-TK Irreversible Inhibitor (ML03): In Vitro and in Vivo Properties, Potential as PET Biomarker for Cancer and Feasibility as Anticancer Drug. Int. J. Cancer 2002, 101, 360–370. [Google Scholar] [CrossRef]
- Mishani, E.; Abourbeh, G.; Rozen, Y.; Jacobson, O.; Laky, D.; Ben David, I.; Levitzki, A.; Shaul, M. Novel carbon-11 labeled 4-dimethylamino-but-2-enoic acid [4-(phenylamino)-quinazoline-6-yl]-amides: Potential PET bioprobes for molecular imaging of EGFR-positive tumors. Nucl. Med. Biol. 2004, 31, 469–476. [Google Scholar] [CrossRef]
- Abourbeh, G.; Dissoki, S.; Jacobson, O.; Litchi, A.; Ben Daniel, R.; Laki, D.; Levitzki, A.; Mishani, E. Evaluation of radiolabeled ML04, a putative irreversible inhibitor of epidermal growth factor receptor, as a bioprobe for PET imaging of EGFR-overexpressing tumors. Nucl. Med. Biol. 2007, 34, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Dissoki, S.; Aviv, Y.; Laky, D.; Abourbeh, G.; Levitzki, A.; Mishani, E. The effect of the [18F]-PEG group on tracer qualification of [4-(phenylamino)-quinazoline-6-YL]-amide moiety—An EGFR putative irreversible inhibitor. Appl. Radiat. Isot. 2007, 65, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Balatoni, J.A.; Mukhopadhyay, U.; Ogawa, K.; Gonzalez-Lepera, C.; Shavrin, A.; Volgin, A.; Tong, W.; Alauddin, M.M.; Gelovani, J.G. Radiosynthesis and initial in vitro evaluation of [18F]F-PEG6-IPQA—A novel PET radiotracer for imaging EGFR expression-activity in lung carcinomas. Mol. Imaging Biol. 2011, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, M.A.; Mishani, E.; Nanni, C.; Landuzzi, L.; Boschi, S.; Nicoletti, G.; Dissoki, S.; Paterini, P.; Piccaluga, P.P.; Lodi, F.; et al. Evaluation of modified PEG-anilinoquinazoline derivatives as potential agents for EGFR imaging in cancer by small animal PET. Mol. Imaging Biol. 2010, 12, 616–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, A.; Glekas, A.; Doubrovin, M.; Balatoni, J.; Namavari, M.; Beresten, T.; Maxwell, D.; Soghomonyan, S.; Shavrin, A.; Ageyeva, L.; et al. Molecular imaging of EGFR kinase activity in tumors with 124I-labeled small molecular tracer and positron emission tomography. Mol. Imaging Biol. 2006, 8, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.H.; Ogawa, K.; Balatoni, J.; Mukhapadhyay, U.; Pal, A.; Gonzalez-Lepera, C.; Shavrin, A.; Soghomonyan, S.; Flores, L., 2nd; Young, D.; et al. Molecular imaging of active mutant L858R EGF receptor (EGFR) kinase-expressing nonsmall cell lung carcinomas using PET/CT. Proc. Natl. Acad. Sci. USA 2011, 108, 1603–1608. [Google Scholar] [CrossRef] [Green Version]
- Quang, T.S.; Brady, L.W. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 972–975. [Google Scholar] [CrossRef]
- Reilly, E.B.; Phillips, A.C.; Buchanan, F.G.; Kingsbury, G.; Zhang, Y.; Meulbroek, J.A.; Cole, T.B.; DeVries, P.J.; Falls, H.D.; Beam, C.; et al. Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody. Mol. Cancer Ther. 2015, 14, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Wehrenberg-Klee, E.; Redjal, N.; Leece, A.; Turker, N.S.; Heidari, P.; Shah, K.; Mahmood, U. PET imaging of glioblastoma multiforme EGFR expression for therapeutic decision guidance. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 379–389. [Google Scholar]
- Cai, W.; Chen, K.; He, L.; Cao, Q.; Koong, A.; Chen, X. Quantitative PET of EGFR expression in xenograft-bearing mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 850–858. [Google Scholar] [CrossRef]
- Van Loon, J.; Even, A.J.G.; Aerts, H.; Öllers, M.; Hoebers, F.; van Elmpt, W.; Dubois, L.; Dingemans, A.C.; Lalisang, R.I.; Kempers, P.; et al. PET imaging of zirconium-89 labelled cetuximab: A phase I trial in patients with head and neck and lung cancer. Radiother. Oncol. 2017, 122, 267–273. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; Gootjes, E.C.; Huisman, M.C.; Vugts, D.J.; Roth, C.; Luik, A.M.; Mulder, E.R.; Schuit, R.C.; Boellaard, R.; Hoekstra, O.S.; et al. 89Zr-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [Google Scholar] [CrossRef] [Green Version]
- Perk, L.R.; Visser, G.W.; Vosjan, M.J.; Stigter-van Walsum, M.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005, 46, 1898–1906. [Google Scholar]
- Song, I.H.; Lee, T.S.; Park, Y.S.; Lee, J.S.; Lee, B.C.; Moon, B.S.; An, G.I.; Lee, H.W.; Kim, K.I.; Lee, Y.J.; et al. Immuno-PET Imaging and Radioimmunotherapy of 64Cu-/177Lu-Labeled Anti-EGFR Antibody in Esophageal Squamous Cell Carcinoma Model. J. Nucl. Med. 2016, 57, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, L.K.; Hoeben, B.A.; Stegeman, H.; Kaanders, J.H.; Franssen, G.M.; Boerman, O.C.; Bussink, J. 111In-cetuximab-F(ab′)2 SPECT imaging for quantification of accessible epidermal growth factor receptors (EGFR) in HNSCC xenografts. Radiother. Oncol. 2013, 108, 484–488. [Google Scholar] [CrossRef]
- Van Dijk, L.K.; Yim, C.B.; Franssen, G.M.; Kaanders, J.H.; Rajander, J.; Solin, O.; Grönroos, T.J.; Boerman, O.C.; Bussink, J. PET of EGFR with (64) Cu-cetuximab-F(ab’)2 in mice with head and neck squamous cell carcinoma xenografts. Contrast Media Mol. Imaging 2016, 11, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Foulon, C.F.; Reist, C.J.; Bigner, D.D.; Zalutsky, M.R. Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 2000, 60, 4453–4460. [Google Scholar]
- Shankar, S.; Vaidyanathan, G.; Affleck, D.J.; Peixoto, K.; Bigner, D.D.; Zalutsky, M.R. Evaluation of an internalizing monoclonal antibody labeled using N-succinimidyl 3-[131I]iodo-4-phosphonomethylbenzoate ([131I]SIPMB), a negatively charged substituent bearing acylation agent. Nucl. Med. Biol. 2004, 31, 909–919. [Google Scholar] [CrossRef]
- Yang, W.; Barth, R.F.; Wu, G.; Kawabata, S.; Sferra, T.J.; Bandyopadhyaya, A.K.; Tjarks, W.; Ferketich, A.K.; Moeschberger, M.L.; Binns, P.J.; et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin. Cancer Res. 2006, 12, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Kuan, C.T.; Reist, C.J.; Foulon, C.F.; Lorimer, I.A.; Archer, G.; Pegram, C.N.; Pastan, I.; Zalutsky, M.R.; Bigner, D.D. 125I-labeled anti-epidermal growth factor receptor-vIII single-chain Fv exhibits specific and high-level targeting of glioma xenografts. Clin. Cancer Res. 1999, 5, 1539–1549. [Google Scholar]
- Miao, Z.; Ren, G.; Liu, H.; Qi, S.; Wu, S.; Cheng, Z. PET of EGFR expression with an 18F-labeled affibody molecule. J. Nucl. Med. 2012, 53, 1110–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikyan, I.; Sundberg, A.L.; Lindhe, O.; Höglund, A.U.; Eriksson, O.; Werner, E.; Carlsson, J.; Bergström, M.; Långström, B.; Tolmachev, V. Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J. Nucl. Med. 2005, 46, 1881–1888. [Google Scholar]
- Pereira, P.M.R.; Norfleet, J.; Lewis, J.S.; Escorcia, F.E. ImmunoPET Detects Changes in Multi-RTK Tumor Cell Expression Levels in Response to Targeted Kinase Inhibition. J. Nucl. Med. 2020, 62, 355–371. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Milenic, D.E.; Brechbiel, M.W. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J. Nucl. Med. 2012, 53, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.J.; De Silva, R.A.; Lapi, S.E. Development and characterization of 89Zr-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging 2013, 12, 17–27. [Google Scholar] [PubMed]
- Lindenberg, L.; Adler, S.; Turkbey, I.B.; Mertan, F.; Ton, A.; Do, K.; Kummar, S.; Gonzalez, E.M.; Bhattacharyya, S.; Jacobs, P.M.; et al. Dosimetry and first human experience with (89)Zr-panitumumab. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 195–203. [Google Scholar] [PubMed]
- Wei, L.; Shi, J.; Afari, G.; Bhattacharyya, S. Preparation of clinical-grade (89) Zr-panitumumab as a positron emission tomography biomarker for evaluating epidermal growth factor receptor-targeted therapy. J. Label. Compd. Radiopharm. 2014, 57, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, R.M.; Kiarash, R.; Cameron, R.G.; Porlier, N.; Sandhu, J.; Hill, R.P.; Vallis, K.; Hendler, A.; Gariépy, J. 111In-labeled EGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J. Nucl. Med. 2000, 41, 429–438. [Google Scholar]
- Li, W.; Niu, G.; Lang, L.; Guo, N.; Ma, Y.; Kiesewetter, D.O.; Backer, J.M.; Shen, B.; Chen, X. PET imaging of EGF receptors using [18F]FBEM-EGF in a head and neck squamous cell carcinoma model. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Sundberg, A.L.; Orlova, A.; Bruskin, A.; Gedda, L.; Carlsson, J.; Blomquist, E.; Lundqvist, H.; Tolmachev, V. [(111)In]Bz-DTPA-hEGF: Preparation and in vitro characterization of a potential anti-glioblastoma targeting agent. Cancer Biother. Radiopharm. 2003, 18, 643–654. [Google Scholar] [CrossRef]
- Denholt, C.L.; Binderup, T.; Stockhausen, M.T.; Poulsen, H.S.; Spang-Thomsen, M.; Hansen, P.R.; Gillings, N.; Kjær, A. Evaluation of 4-[18F]fluorobenzoyl-FALGEA-NH2 as a positron emission tomography tracer for epidermal growth factor receptor mutation variant III imaging in cancer. Nucl. Med. Biol. 2011, 38, 509–515. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of Aptamer to U87-EGFRvIII Cells on the Proliferation, Radiosensitivity, and Radiotherapy of Glioblastoma Cells. Mol. Ther. Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Turner, S.; Peters, K.B.; Desjardins, A.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E., 2nd; Jones, L.W.; Kirkpatrick, J.P.; et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J. Natl. Compr. Cancer Netw. 2011, 9, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef]
- Chinot, O.L.; de La Motte Rouge, T.; Moore, N.; Zeaiter, A.; Das, A.; Phillips, H.; Modrusan, Z.; Cloughesy, T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 2011, 28, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [Green Version]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Kalpathy-Cramer, J.; Chandra, V.; Da, X.; Ou, Y.; Emblem, K.E.; Muzikansky, A.; Cai, X.; Douw, L.; Evans, J.G.; Dietrich, J.; et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J. Neurooncol. 2017, 131, 603–610. [Google Scholar] [CrossRef]
- De Groot, J.F.; Lamborn, K.R.; Chang, S.M.; Gilbert, M.R.; Cloughesy, T.F.; Aldape, K.; Yao, J.; Jackson, E.F.; Lieberman, F.; Robins, H.I.; et al. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium study. J. Clin. Oncol. 2011, 29, 2689–2695. [Google Scholar] [CrossRef]
- Nayak, L.; de Groot, J.; Wefel, J.S.; Cloughesy, T.F.; Lieberman, F.; Chang, S.M.; Omuro, A.; Drappatz, J.; Batchelor, T.T.; DeAngelis, L.M.; et al. Phase I trial of aflibercept (VEGF trap) with radiation therapy and concomitant and adjuvant temozolomide in patients with high-grade gliomas. J. Neurooncol. 2017, 132, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, E.R.; Eichler, A.F.; Plotkin, S.R.; Drappatz, J.; Doyle, C.L.; Xu, L.; Duda, D.G.; Wen, P.Y.; Jain, R.K.; Batchelor, T.T. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J. Neurooncol. 2011, 103, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandes, A.A.; Stupp, R.; Hau, P.; Lacombe, D.; Gorlia, T.; Tosoni, A.; Mirimanoff, R.O.; Kros, J.M.; van den Bent, M.J. EORTC study 26041-22041: Phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur. J. Cancer 2010, 46, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Egorin, M.J.; Desjardins, A.; Vredenburgh, J.J.; Beumer, J.H.; Lagattuta, T.F.; Gururangan, S.; Herndon, J.E., 2nd; Salvado, A.J.; Friedman, H.S. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer 2009, 115, 2188–2198. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Plotkin, S.R.; Gerstner, E.; Eichler, A.F.; Drappatz, J.; Hochberg, F.H.; Benner, T.; et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010, 28, 2817–2823. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Awada, G.; Ben Salama, L.; De Cremer, J.; Schwarze, J.K.; Fischbuch, L.; Seynaeve, L.; Du Four, S.; Vanbinst, A.M.; Michotte, A.; Everaert, H.; et al. Axitinib plus avelumab in the treatment of recurrent glioblastoma: A stratified, open-label, single-center phase 2 clinical trial (GliAvAx). J. Immunother. Cancer 2020, 8, e001146. [Google Scholar] [CrossRef]
- Duerinck, J.; Du Four, S.; Vandervorst, F.; D’Haene, N.; Le Mercier, M.; Michotte, A.; Van Binst, A.M.; Everaert, H.; Salmon, I.; Bouttens, F.; et al. Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J. Neurooncol. 2016, 128, 147–155. [Google Scholar] [CrossRef]
- Duerinck, J.; Du Four, S.; Bouttens, F.; Andre, C.; Verschaeve, V.; Van Fraeyenhove, F.; Chaskis, C.; D’Haene, N.; Le Mercier, M.; Rogiers, A.; et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J. Neurooncol. 2018, 136, 115–125. [Google Scholar] [CrossRef]
- Wick, W.; Puduvalli, V.K.; Chamberlain, M.C.; van den Bent, M.J.; Carpentier, A.F.; Cher, L.M.; Mason, W.; Weller, M.; Hong, S.; Musib, L.; et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010, 28, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- De Groot, J.F.; Piao, Y.; Tran, H.; Gilbert, M.; Wu, H.K.; Liu, J.; Bekele, B.N.; Cloughesy, T.; Mehta, M.; Robins, H.I.; et al. Myeloid biomarkers associated with glioblastoma response to anti-VEGF therapy with aflibercept. Clin. Cancer Res. 2011, 17, 4872–4881. [Google Scholar] [CrossRef] [Green Version]
- Wirsching, H.G.; Roth, P.; Weller, M. A vasculature-centric approach to developing novel treatment options for glioblastoma. Expert Opin. Ther. Targets 2021, 25, 87–100. [Google Scholar] [CrossRef]
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968. [Google Scholar] [CrossRef] [Green Version]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef]
- Raizer, J.J.; Grimm, S.; Chamberlain, M.C.; Nicholas, M.K.; Chandler, J.P.; Muro, K.; Dubner, S.; Rademaker, A.W.; Renfrow, J.; Bredel, M. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 2010, 116, 5297–5305. [Google Scholar] [CrossRef]
- Nagane, M.; Nishikawa, R.; Narita, Y.; Kobayashi, H.; Takano, S.; Shinoura, N.; Aoki, T.; Sugiyama, K.; Kuratsu, J.; Muragaki, Y.; et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. JPN J. Clin. Oncol. 2012, 42, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Botero, G.; Cartalat-Carel, S.; Chinot, O.L.; Barrie, M.; Taillandier, L.; Beauchesne, P.; Catry-Thomas, I.; Barrière, J.; Guillamo, J.S.; Fabbro, M.; et al. Temozolomide Plus Bevacizumab in Elderly Patients with Newly Diagnosed Glioblastoma and Poor Performance Status: An ANOCEF Phase II Trial (ATAG). Oncologist 2018, 23, e524–e544. [Google Scholar] [CrossRef] [Green Version]
- Balana, C.; De Las Penas, R.; Sepúlveda, J.M.; Gil-Gil, M.J.; Luque, R.; Gallego, O.; Carrato, C.; Sanz, C.; Reynes, G.; Herrero, A.; et al. Bevacizumab and temozolomide versus temozolomide alone as neoadjuvant treatment in unresected glioblastoma: The GENOM 009 randomized phase II trial. J. Neurooncol. 2016, 127, 569–579. [Google Scholar] [CrossRef]
- Lou, E.; Peters, K.B.; Sumrall, A.L.; Desjardins, A.; Reardon, D.A.; Lipp, E.S.; Herndon, J.E., 2nd; Coan, A.; Bailey, L.; Turner, S.; et al. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2013, 2, 185–195. [Google Scholar] [CrossRef]
- Desjardins, A.; Reardon, D.A.; Coan, A.; Marcello, J.; Herndon, J.E., 2nd; Bailey, L.; Peters, K.B.; Friedman, H.S.; Vredenburgh, J.J. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 2012, 118, 1302–1312. [Google Scholar] [CrossRef]
- Wirsching, H.G.; Tabatabai, G.; Roelcke, U.; Hottinger, A.F.; Jörger, F.; Schmid, A.; Plasswilm, L.; Schrimpf, D.; Mancao, C.; Capper, D.; et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with glioblastoma: The randomized, open-label, phase II ARTE trial. Ann. Oncol. 2018, 29, 1423–1430. [Google Scholar] [CrossRef]
- Van Linde, M.E.; Verhoeff, J.J.; Richel, D.J.; van Furth, W.R.; Reijneveld, J.C.; Verheul, H.M.; Stalpers, L.J. Bevacizumab in combination with radiotherapy and temozolomide for patients with newly diagnosed glioblastoma multiforme. Oncologist 2015, 20, 107–108. [Google Scholar] [CrossRef] [Green Version]
- Narayana, A.; Gruber, D.; Kunnakkat, S.; Golfinos, J.G.; Parker, E.; Raza, S.; Zagzag, D.; Eagan, P.; Gruber, M.L. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J. Neurosurg. 2012, 116, 341–345. [Google Scholar] [CrossRef]
- Lai, A.; Tran, A.; Nghiemphu, P.L.; Pope, W.B.; Solis, O.E.; Selch, M.; Filka, E.; Yong, W.H.; Mischel, P.S.; Liau, L.M.; et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2011, 29, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Omuro, A.; Beal, K.; Gutin, P.; Karimi, S.; Correa, D.D.; Kaley, T.J.; DeAngelis, L.M.; Chan, T.A.; Gavrilovic, I.T.; Nolan, C.; et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin. Cancer Res. 2014, 20, 5023–5031. [Google Scholar] [CrossRef] [Green Version]
- Ney, D.E.; Carlson, J.A.; Damek, D.M.; Gaspar, L.E.; Kavanagh, B.D.; Kleinschmidt-DeMasters, B.K.; Waziri, A.E.; Lillehei, K.O.; Reddy, K.; Chen, C. Phase II trial of hypofractionated intensity-modulated radiation therapy combined with temozolomide and bevacizumab for patients with newly diagnosed glioblastoma. J. Neurooncol. 2015, 122, 135–143. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Reardon, D.A.; Peters, K.B.; Herndon, J.E., 2nd; Marcello, J.; Kirkpatrick, J.P.; Sampson, J.H.; Bailey, L.; Threatt, S.; et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin. Cancer Res. 2011, 17, 4119–4124. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.K.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauffert, B.; Feuvret, L.; Bonnetain, F.; Taillandier, L.; Frappaz, D.; Taillia, H.; Schott, R.; Honnorat, J.; Fabbro, M.; Tennevet, I.; et al. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEF. Ann. Oncol. 2014, 25, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.B.; Lou, E.; Desjardins, A.; Reardon, D.A.; Lipp, E.S.; Miller, E.; Herndon, J.E., 2nd; McSherry, F.; Friedman, H.S.; Vredenburgh, J.J. Phase II Trial of Upfront Bevacizumab, Irinotecan, and Temozolomide for Unresectable Glioblastoma. Oncologist 2015, 20, 727–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrlinger, U.; Schäfer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine-DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Pugh, S.L.; Aldape, K.; Sorensen, A.G.; Mikkelsen, T.; Penas-Prado, M.; Bokstein, F.; Kwok, Y.; Lee, R.J.; Mehta, M. NRG oncology RTOG 0625: A randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J. Neurooncol. 2017, 131, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Hofland, K.F.; Hansen, S.; Sorensen, M.; Engelholm, S.; Schultz, H.P.; Muhic, A.; Grunnet, K.; Ask, A.; Costa, J.C.; Kristiansen, C.; et al. Neoadjuvant bevacizumab and irinotecan versus bevacizumab and temozolomide followed by concomitant chemoradiotherapy in newly diagnosed glioblastoma multiforme: A randomized phase II study. Acta Oncol. 2014, 53, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Brandes, A.A.; Gil-Gil, M.; Saran, F.; Carpentier, A.F.; Nowak, A.K.; Mason, W.; Zagonel, V.; Dubois, F.; Finocchiaro, G.; Fountzilas, G.; et al. A Randomized Phase II Trial (TAMIGA) Evaluating the Efficacy and Safety of Continuous Bevacizumab Through Multiple Lines of Treatment for Recurrent Glioblastoma. Oncologist 2019, 24, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [Google Scholar] [CrossRef]
- Weathers, S.P.; Han, X.; Liu, D.D.; Conrad, C.A.; Gilbert, M.R.; Loghin, M.E.; O’Brien, B.J.; Penas-Prado, M.; Puduvalli, V.K.; Tremont-Lukats, I.; et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J. Neurooncol. 2016, 129, 487–494. [Google Scholar] [CrossRef]
- Field, K.M.; Simes, J.; Nowak, A.K.; Cher, L.; Wheeler, H.; Hovey, E.J.; Brown, C.S.; Barnes, E.H.; Sawkins, K.; Livingstone, A.; et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015, 17, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Desjardins, A.; Peters, K.B.; Gururangan, S.; Sampson, J.H.; McLendon, R.E.; Herndon, J.E., 2nd; Bulusu, A.; Threatt, S.; Friedman, A.H.; et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naïve, recurrent glioblastoma. J. Neurooncol. 2012, 107, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Hainsworth, J.D.; Becker, K.P.; Mekhail, T.; Chowdhary, S.A.; Eakle, J.F.; Wright, D.; Langdon, R.M.; Yost, K.J.; Padula, G.D.A.; West-Osterfield, K.; et al. Phase I/II study of bevacizumab with BKM120, an oral PI3K inhibitor, in patients with refractory solid tumors (phase I) and relapsed/refractory glioblastoma (phase II). J. Neurooncol. 2019, 144, 303–311. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Twohy, E.L.; Carrero, X.W.; Dixon, J.G.; Tran, D.D.; Jeyapalan, S.A.; Anderson, D.M.; Kaufmann, T.J.; Feathers, R.W.; et al. A phase 1 and randomized, placebo-controlled phase 2 trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma: Alliance/North Central Cancer Treatment Group N0872. Cancer 2019, 125, 3790–3800. [Google Scholar] [CrossRef]
- Bota, D.A.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.T.; Fu, B.D.; Hsu, F.P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4(+) T-lymphocyte counts. CNS Oncol. 2018, 7, Cns22. [Google Scholar] [CrossRef] [Green Version]
- Cloughesy, T.; Finocchiaro, G.; Belda-Iniesta, C.; Recht, L.; Brandes, A.A.; Pineda, E.; Mikkelsen, T.; Chinot, O.L.; Balana, C.; Macdonald, D.R.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O(6)-Methylguanine-DNA Methyltransferase Biomarker Analyses. J. Clin. Oncol. 2017, 35, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Lassen, U.; Sorensen, M.; Gaziel, T.B.; Hasselbalch, B.; Poulsen, H.S. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013, 33, 1657–1660. [Google Scholar]
- Odia, Y.; Sul, J.; Shih, J.H.; Kreisl, T.N.; Butman, J.A.; Iwamoto, F.M.; Fine, H.A. A Phase II trial of tandutinib (MLN 518) in combination with bevacizumab for patients with recurrent glioblastoma. CNS Oncol. 2016, 5, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Brandes, A.A.; Finocchiaro, G.; Zagonel, V.; Reni, M.; Caserta, C.; Fabi, A.; Clavarezza, M.; Maiello, E.; Eoli, M.; Lombardi, G.; et al. AVAREG: A phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol. 2016, 18, 1304–1312. [Google Scholar] [CrossRef]
- Soffietti, R.; Trevisan, E.; Bertero, L.; Cassoni, P.; Morra, I.; Fabrini, M.G.; Pasqualetti, F.; Lolli, I.; Castiglione, A.; Ciccone, G.; et al. Bevacizumab and fotemustine for recurrent glioblastoma: A phase II study of AINO (Italian Association of Neuro-Oncology). J. Neurooncol. 2014, 116, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Hainsworth, J.D.; Shih, K.C.; Shepard, G.C.; Tillinghast, G.W.; Brinker, B.T.; Spigel, D.R. Phase II study of concurrent radiation therapy, temozolomide, and bevacizumab followed by bevacizumab/everolimus as first-line treatment for patients with glioblastoma. Clin. Adv. Hematol. Oncol. 2012, 10, 240–246. [Google Scholar]
- Reardon, D.A.; Desjardins, A.; Peters, K.; Gururangan, S.; Sampson, J.; Rich, J.N.; McLendon, R.; Herndon, J.E., 2nd; Marcello, J.; Threatt, S.; et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J. Neurooncol. 2011, 103, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Q.; Reardon, D.A.; Schiff, D.; Drappatz, J.; Muzikansky, A.; Grimm, S.A.; Norden, A.D.; Nayak, L.; Beroukhim, R.; Rinne, M.L.; et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015, 17, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.; Neil, E.; Terziev, R.; Gutin, P.; Barani, I.; Kaley, T.; Lassman, A.B.; Chan, T.A.; Yamada, J.; DeAngelis, L.; et al. Multicenter, Phase 1, Dose Escalation Study of Hypofractionated Stereotactic Radiation Therapy with Bevacizumab for Recurrent Glioblastoma and Anaplastic Astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 797–804. [Google Scholar] [CrossRef]
- Galanis, E.; Anderson, S.K.; Lafky, J.M.; Uhm, J.H.; Giannini, C.; Kumar, S.K.; Kimlinger, T.K.; Northfelt, D.W.; Flynn, P.J.; Jaeckle, K.A.; et al. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A north central cancer treatment group trial. Clin. Cancer Res. 2013, 19, 4816–4823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathornsumetee, S.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Marcello, J.; Herndon, J.E.; Mathe, A.; Hamilton, M.; Rich, J.N.; Norfleet, J.A.; et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010, 12, 1300–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiaseddin, A.; Reardon, D.; Massey, W.; Mannerino, A.; Lipp, E.S.; Herndon, J.E., 2nd; McSherry, F.; Desjardins, A.; Randazzo, D.; Friedman, H.S.; et al. Phase II Study of Bevacizumab and Vorinostat for Patients with Recurrent World Health Organization Grade 4 Malignant Glioma. Oncologist 2018, 23, 157-e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, K.B.; Lipp, E.S.; Miller, E.; Herndon, J.E., 2nd; McSherry, F.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J. Neurooncol. 2018, 137, 349–356. [Google Scholar] [CrossRef]
- Odia, Y.; Iwamoto, F.M.; Moustakas, A.; Fraum, T.J.; Salgado, C.A.; Li, A.; Kreisl, T.N.; Sul, J.; Butman, J.A.; Fine, H.A. A phase II trial of enzastaurin (LY317615) in combination with bevacizumab in adults with recurrent malignant gliomas. J. Neurooncol. 2016, 127, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Thompson, G.; Mills, S.J.; Coope, D.J.; O’Connor, J.P.; Jackson, A. Imaging biomarkers of angiogenesis and the microvascular environment in cerebral tumours. Br. J. Radiol. 2011, 84, S127–S144. [Google Scholar] [CrossRef] [Green Version]
- Gaykema, S.B.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef] [Green Version]
- Oosting, S.F.; Brouwers, A.H.; van Es, S.C.; Nagengast, W.B.; Oude Munnink, T.H.; Lub-de Hooge, M.N.; Hollema, H.; de Jong, J.R.; de Jong, I.J.; de Haas, S.; et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 2015, 56, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M.H.; Lagerweij, T.; Sewing, A.C.; Vugts, D.J.; van Vuurden, D.G.; Molthoff, C.F.; Caretti, V.; Veringa, S.J.; Petersen, N.; Carcaboso, A.M.; et al. Bevacizumab Targeting Diffuse Intrinsic Pontine Glioma: Results of 89Zr-Bevacizumab PET Imaging in Brain Tumor Models. Mol. Cancer Ther. 2016, 15, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Rainer, E.; Wang, H.; Traub-Weidinger, T.; Widhalm, G.; Fueger, B.; Chang, J.; Zhu, Z.; Marosi, C.; Haug, A.; Hacker, M.; et al. The prognostic value of [123I]-vascular endothelial growth factor ([(123I]-VEGF) in glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2396–2403. [Google Scholar] [CrossRef] [Green Version]
- Laffon, E.; Marthan, R. A three-time-point method for assessing kinetic parameters of (64)Cu-labeled Ramucirumab trapping in VEGFR-2 positive lung tumors. Phys. Med. 2017, 43, 1–5. [Google Scholar] [CrossRef]
- Luo, H.; England, C.G.; Graves, S.A.; Sun, H.; Liu, G.; Nickles, R.J.; Cai, W. PET Imaging of VEGFR-2 Expression in Lung Cancer with 64Cu-Labeled Ramucirumab. J. Nucl. Med. 2016, 57, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Nagengast, W.B.; Lub-de Hooge, M.N.; Oosting, S.F.; den Dunnen, W.F.; Warnders, F.J.; Brouwers, A.H.; de Jong, J.R.; Price, P.M.; Hollema, H.; Hospers, G.A.; et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res. 2011, 71, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Kameswaran, M.; Sarma, H.D.; Dash, A. Preclinical evaluation of (131)I-Bevacizumab—A prospective agent for radioimmunotherapy in VEGF expressing cancers. Appl. Radiat. Isot. 2017, 123, 109–113. [Google Scholar] [CrossRef]
- Stollman, T.H.; Scheer, M.G.; Leenders, W.P.; Verrijp, K.C.; Soede, A.C.; Oyen, W.J.; Ruers, T.J.; Boerman, O.C. Specific imaging of VEGF-A expression with radiolabeled anti-VEGF monoclonal antibody. Int. J. Cancer 2008, 122, 2310–2314. [Google Scholar] [CrossRef]
- Mitran, B.; Güler, R.; Roche, F.P.; Lindström, E.; Selvaraju, R.K.; Fleetwood, F.; Rinne, S.S.; Claesson-Welsh, L.; Tolmachev, V.; Ståhl, S.; et al. Radionuclide imaging of VEGFR2 in glioma vasculature using biparatopic affibody conjugate: Proof-of-principle in a murine model. Theranostics 2018, 8, 4462–4476. [Google Scholar] [CrossRef]
- Chan, C.; Sandhu, J.; Guha, A.; Scollard, D.A.; Wang, J.; Chen, P.; Bai, K.; Lee, L.; Reilly, R.M. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for (111)In for imaging tumor angiogenesis. J. Nucl. Med. 2005, 46, 1745–1752. [Google Scholar]
- Cai, W.; Chen, K.; Mohamedali, K.A.; Cao, Q.; Gambhir, S.S.; Rosenblum, M.G.; Chen, X. PET of vascular endothelial growth factor receptor expression. J. Nucl. Med. 2006, 47, 2048–2056. [Google Scholar]
- Hu, K.; Shang, J.; Xie, L.; Hanyu, M.; Zhang, Y.; Yang, Z.; Xu, H.; Wang, L.; Zhang, M.R. PET Imaging of VEGFR with a Novel (64)Cu-Labeled Peptide. ACS Omega 2020, 5, 8508–8514. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Cai, W.; Li, Z.B.; Wang, H.; Chen, X. Quantitative PET imaging of VEGF receptor expression. Mol. Imaging Biol. 2009, 11, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Guo, D. MET in glioma: Signaling pathways and targeted therapies. J. Exp. Clin. Cancer Res. 2019, 38, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, D.S.; Song, S.Y.; Kim, D.H.; Joo, K.M.; Yoo, J.S.; Koh, J.S.; Dong, S.M.; Suh, Y.L.; Lee, J.I.; Park, K.; et al. Prognostic significance of c-Met expression in glioblastomas. Cancer 2009, 115, 140–148. [Google Scholar] [CrossRef]
- Mulcahy, E.Q.X.; Colόn, R.R.; Abounader, R. HGF/MET Signaling in Malignant Brain Tumors. Int. J. Mol. Sci. 2020, 21, 7546. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, N.; Zhang, Y.; Hine, S.; Gibert, M.; Yuan, F.; Oxford, M.; Grello, C.; Pahuski, M.; Dube, C.; Guessous, F.; et al. Discovery and Therapeutic Exploitation of Mechanisms of Resistance to MET Inhibitors in Glioblastoma. Clin. Cancer Res. 2019, 25, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Cruickshanks, N.; Zhang, Y.; Yuan, F.; Pahuski, M.; Gibert, M.; Abounader, R. Role and Therapeutic Targeting of the HGF/MET Pathway in Glioblastoma. Cancers 2017, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.Y.; Schiff, D.; Cloughesy, T.F.; Raizer, J.J.; Laterra, J.; Smitt, M.; Wolf, M.; Oliner, K.S.; Anderson, A.; Zhu, M.; et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011, 13, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affronti, M.L.; Jackman, J.G.; McSherry, F.; Herndon, J.E., 2nd; Massey, E.C., Jr.; Lipp, E.; Desjardins, A.; Friedman, H.S.; Vlahovic, G.; Vredenburgh, J.; et al. Phase II Study to Evaluate the Efficacy and Safety of Rilotumumab and Bevacizumab in Subjects with Recurrent Malignant Glioma. Oncologist 2018, 23, e889–e898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Dhillon, S. Capmatinib: First Approval. Drugs 2020, 80, 1125–1131. [Google Scholar] [CrossRef]
- Van den Bent, M.; Azaro, A.; De Vos, F.; Sepulveda, J.; Yung, W.K.A.; Wen, P.Y.; Lassman, A.B.; Joerger, M.; Tabatabai, G.; Rodon, J.; et al. A Phase Ib/II, open-label, multicenter study of INC280 (capmatinib) alone and in combination with buparlisib (BKM120) in adult patients with recurrent glioblastoma. J. Neurooncol. 2020, 146, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Mu, Q.; Bao, Z.; Chen, Y.; Liu, Y.; Chen, J.; Wang, K.; Wang, Z.; Nam, Y.; Jiang, B.; et al. Mutational Landscape of Secondary Glioblastoma Guides MET-Targeted Trial in Brain Tumor. Cell 2018, 175, 1665–1678.e18. [Google Scholar] [CrossRef] [Green Version]
- Broniscer, A.; Jia, S.; Mandrell, B.; Hamideh, D.; Huang, J.; Onar-Thomas, A.; Gajjar, A.; Raimondi, S.C.; Tatevossian, R.G.; Stewart, C.F. Phase 1 trial, pharmacokinetics, and pharmacodynamics of dasatinib combined with crizotinib in children with recurrent or progressive high-grade and diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2018, 65, e27035. [Google Scholar] [CrossRef]
- Guessous, F.; Zhang, Y.; diPierro, C.; Marcinkiewicz, L.; Sarkaria, J.; Schiff, D.; Buchanan, S.; Abounader, R. An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anticancer Agents Med. Chem. 2010, 10, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Hong, S.H.; Kim, J.Y.; Kim, I.C.; Park, Y.W.; Lee, S.J.; Song, S.W.; Kim, J.J.; Park, G.; Kim, T.M.; et al. Preclinical development of a humanized neutralizing antibody targeting HGF. Exp. Mol. Med. 2017, 49, e309. [Google Scholar] [CrossRef]
- Sa, J.K.; Kim, S.H.; Lee, J.K.; Cho, H.J.; Shin, Y.J.; Shin, H.; Koo, H.; Kim, D.; Lee, M.; Kang, W.; et al. Identification of genomic and molecular traits that present therapeutic vulnerability to HGF-targeted therapy in glioblastoma. Neuro Oncol. 2019, 21, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Piao, Y.; Park, S.Y.; Henry, V.; Smith, B.D.; Tiao, N.; Flynn, D.L.; de Groot, J.F. Novel MET/TIE2/VEGFR2 inhibitor altiratinib inhibits tumor growth and invasiveness in bevacizumab-resistant glioblastoma mouse models. Neuro Oncol. 2016, 18, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Knubel, K.H.; Pernu, B.M.; Sufit, A.; Nelson, S.; Pierce, A.M.; Keating, A.K. MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget 2014, 5, 1338–1351. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Shu, M.; Chen, Y.; Yang, D.; He, Q.; Zhao, H.; Feng, Z.; Liang, C.; Yu, K. A novel lead compound CM-118: Antitumor activity and new insight into the molecular mechanism and combination therapy strategy in c-Met- and ALK-dependent cancers. Cancer Biol. Ther. 2014, 15, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Dai, G.; Weng, J.; Zhang, Z.; Wang, Q.; Zhou, F.; Jiao, L.; Cui, Y.; Ren, Y.; Fan, S.; et al. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J. Med. Chem. 2014, 57, 7577–7589. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Yang, G.; Marando, C.; Koblish, H.K.; Hall, L.M.; Fridman, J.S.; Behshad, E.; Wynn, R.; Li, Y.; et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin. Cancer Res. 2011, 17, 7127–7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Li, Z.; Zhang, L.; Liu, G. Tivantinib Hampers the Proliferation of Glioblastoma Cells via PI3K/Akt/Mammalian Target of Rapamycin (mTOR) Signaling. Med. Sci. Monit. 2019, 25, 7383–7390. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Narayan, R.N.; Horton, L.; Patel, T.R.; Habib, A.A. The Role of EGFR-Met Interactions in the Pathogenesis of Glioblastoma and Resistance to Treatment. Curr. Cancer Drug Targets 2017, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; De Lay, M.; Miller, L.M.; Carbonell, W.S.; Hu, Y.L.; Lu, K.; Tom, M.W.; Paquette, J.; Tokuyasu, T.A.; Tsao, S.; et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin. Cancer Res. 2013, 19, 1773–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.V.; Chang, J.P.; Parachoniak, C.A.; Pandika, M.M.; Aghi, M.K.; Meyronet, D.; Isachenko, N.; Fouse, S.D.; Phillips, J.J.; Cheresh, D.A.; et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012, 22, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H.J.; Acquaviva, J.; Chi, D.; Lessard, J.; Zhu, H.; Woolfenden, S.; Bronson, R.T.; Pfannl, R.; White, F.; Housman, D.E.; et al. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene 2012, 31, 3039–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Xiao, Y.; Wang, K.; Yan, J.; Xiao, Z.; Fang, F.; Jin, Z.; Liu, Y.; Sun, X.; Shen, B. Development of a SPECT Tracer to Image c-Met Expression in a Xenograft Model of Non-Small Cell Lung Cancer. J. Nucl. Med. 2018, 59, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, B.S.; Zhao, P.; Resau, J.; Cottingham, S.; Gherardi, E.; Xu, E.; Berghuis, B.; Daugherty, J.; Grabinski, T.; Toro, J.; et al. A novel multipurpose monoclonal antibody for evaluating human c-Met expression in preclinical and clinical settings. Appl. Immunohistochem. Mol. Morphol. 2009, 17, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Wu, Y.; Wang, K.; Xiao, Y.; Cheng, Z.; Sun, X.; Shen, B. Analysis of progress and challenges for various patterns of c-MET-targeted molecular imaging: A systematic review. EJNMMI Res. 2017, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Hong, H.; Slater, M.R.; Graves, S.A.; Shi, S.; Yang, Y.; Nickles, R.J.; Fan, F.; Cai, W. PET of c-Met in Cancer with 64Cu-Labeled Hepatocyte Growth Factor. J. Nucl. Med. 2015, 56, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Pool, M.; Terwisscha van Scheltinga, A.G.T.; Kol, A.; Giesen, D.; de Vries, E.G.E.; Lub-de Hooge, M.N. (89)Zr-Onartuzumab PET imaging of c-MET receptor dynamics. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Jagoda, E.M.; Lang, L.; Bhadrasetty, V.; Histed, S.; Williams, M.; Kramer-Marek, G.; Mena, E.; Rosenblum, L.; Marik, J.; Tinianow, J.N.; et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J. Nucl. Med. 2012, 53, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Price, E.W.; Carnazza, K.E.; Carlin, S.D.; Cho, A.; Edwards, K.J.; Sevak, K.K.; Glaser, J.M.; de Stanchina, E.; Janjigian, Y.Y.; Lewis, J.S. (89)Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection. J. Nucl. Med. 2017, 58, 1386–1394. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.M.; Park, E.H.; Cheong, S.J.; Lee, C.M.; Kim, D.W.; Jeong, H.J.; Lim, S.T.; Sohn, M.H.; Kim, K.; Chung, J. Characterization, biodistribution and small-animal SPECT of I-125-labeled c-Met binding peptide in mice bearing c-Met receptor tyrosine kinase-positive tumor xenografts. Nucl. Med. Biol 2009, 36, 371–378. [Google Scholar] [CrossRef]
- Kim, E.M.; Jeong, M.H.; Kim, D.W.; Jeong, H.J.; Lim, S.T.; Sohn, M.H. Iodine 125-labeled mesenchymal-epithelial transition factor binding peptide-click-cRGDyk heterodimer for glioma imaging. Cancer Sci. 2011, 102, 1516–1521. [Google Scholar] [CrossRef]
- Arulappu, A.; Battle, M.; Eisenblaetter, M.; McRobbie, G.; Khan, I.; Monypenny, J.; Weitsman, G.; Galazi, M.; Hoppmann, S.; Gazinska, P.; et al. c-Met PET Imaging Detects Early-Stage Locoregional Recurrence of Basal-Like Breast Cancer. J. Nucl. Med. 2016, 57, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Fomchenko, E.I.; Holland, E.C. Platelet-derived growth factor-mediated gliomagenesis and brain tumor recruitment. Neurosurg. Clin. N. Am. 2007, 18, 39–58. [Google Scholar] [CrossRef]
- Nazarenko, I.; Hede, S.M.; He, X.; Hedrén, A.; Thompson, J.; Lindström, M.S.; Nistér, M. PDGF and PDGF receptors in glioma. Upsala J. Med. Sci. 2012, 117, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Cantanhede, I.G.; de Oliveira, J.R.M. PDGF Family Expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci. Rep. 2017, 7, 15271. [Google Scholar] [CrossRef] [Green Version]
- Szerlip, N.J.; Pedraza, A.; Chakravarty, D.; Azim, M.; McGuire, J.; Fang, Y.; Ozawa, T.; Holland, E.C.; Huse, J.T.; Jhanwar, S.; et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc. Natl. Acad. Sci. USA 2012, 109, 3041–3046. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, E.; Wu, Q.; Guryanova, O.; Hitomi, M.; Lathia, J.D.; Serwanski, D.; Sloan, A.E.; Weil, R.J.; Lee, J.; et al. Platelet-derived growth factor receptors differentially inform intertumoral and intratumoral heterogeneity. Genes Dev. 2012, 26, 1247–1262. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, T.T.; Gerstner, E.R.; Ye, X.; Desideri, S.; Duda, D.G.; Peereboom, D.; Lesser, G.J.; Chowdhary, S.; Wen, P.Y.; Grossman, S.; et al. Feasibility, phase I, and phase II studies of tandutinib, an oral platelet-derived growth factor receptor-β tyrosine kinase inhibitor, in patients with recurrent glioblastoma. Neuro Oncol. 2017, 19, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Picconi, D.; Juarez, T.; Kesari, S. ACTR-56. Phase II trial of nilotinib in PDGFR-alpha enriched recurrent glioblastoma. Neuro Oncol. 2019, 21, vi26. [Google Scholar] [CrossRef]
- Alexandru, O.; Sevastre, A.S.; Castro, J.; Artene, S.A.; Tache, D.E.; Purcaru, O.S.; Sfredel, V.; Tataranu, L.G.; Dricu, A. Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. Int. J. Mol. Sci. 2019, 20, 4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, A.M.; Alexandru, O.; Brindusa, C.; Purcaru, S.O.; Tache, D.E.; Tataranu, L.G.; Taisescu, C.; Dricu, A. Targeting the VEGF and PDGF signaling pathway in glioblastoma treatment. Int. J. Clin. Exp. Pathol. 2015, 8, 7825–7837. [Google Scholar] [PubMed]
- Ziegler, D.S.; Wright, R.D.; Kesari, S.; Lemieux, M.E.; Tran, M.A.; Jain, M.; Zawel, L.; Kung, A.L. Resistance of human glioblastoma multiforme cells to growth factor inhibitors is overcome by blockade of inhibitor of apoptosis proteins. J. Clin. Investig. 2008, 118, 3109–3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokker, N.A.; Sullivan, C.M.; Hollenbach, S.J.; Israel, M.A.; Giese, N.A. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002, 62, 3729–3735. [Google Scholar] [PubMed]
- Roberts, W.G.; Whalen, P.M.; Soderstrom, E.; Moraski, G.; Lyssikatos, J.P.; Wang, H.F.; Cooper, B.; Baker, D.A.; Savage, D.; Dalvie, D.; et al. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res. 2005, 65, 957–966. [Google Scholar] [PubMed]
- Kil, K.E.; Ding, Y.S.; Lin, K.S.; Alexoff, D.; Kim, S.W.; Shea, C.; Xu, Y.; Muench, L.; Fowler, J.S. Synthesis and positron emission tomography studies of carbon-11-labeled imatinib (Gleevec). Nucl. Med. Biol. 2007, 34, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, V.; Varasteh, Z.; Honarvar, H.; Hosseinimehr, S.J.; Eriksson, O.; Jonasson, P.; Frejd, F.Y.; Abrahmsen, L.; Orlova, A. Imaging of platelet-derived growth factor receptor β expression in glioblastoma xenografts using affibody molecule 111In-DOTA-Z09591. J. Nucl. Med. 2014, 55, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Strand, J.; Varasteh, Z.; Eriksson, O.; Abrahmsen, L.; Orlova, A.; Tolmachev, V. Gallium-68-labeled affibody molecule for PET imaging of PDGFRβ expression in vivo. Mol. Pharm. 2014, 11, 3957–3964. [Google Scholar] [CrossRef]
- Effendi, N.; Mishiro, K.; Takarada, T.; Makino, A.; Yamada, D.; Kitamura, Y.; Shiba, K.; Kiyono, Y.; Odani, A.; Ogawa, K. Radiobrominated benzimidazole-quinoline derivatives as Platelet-derived growth factor receptor beta (PDGFRβ) imaging probes. Sci. Rep. 2018, 8, 10369. [Google Scholar] [CrossRef]
- Effendi, N.; Ogawa, K.; Mishiro, K.; Takarada, T.; Yamada, D.; Kitamura, Y.; Shiba, K.; Maeda, T.; Odani, A. Synthesis and evaluation of radioiodinated 1-{2-[5-(2-methoxyethoxy)-1H-benzo[d]imidazol-1-yl]quinolin-8-yl}piperidin-4-amine derivatives for platelet-derived growth factor receptor β (PDGFRβ) imaging. Bioorg. Med. Chem. 2017, 25, 5576–5585. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Wuest, M.; Hamann, I.; Lopez-Campistrous, A.; McMullen, T.P.W.; Wuest, F. Molecular imaging of platelet-derived growth factor receptor-alpha (PDGFRα) in papillary thyroid cancer using immuno-PET. Nucl. Med. Biol. 2018, 58, 51–58. [Google Scholar] [CrossRef]
- Effendi, N.; Mishiro, K.; Shiba, K.; Kinuya, S.; Ogawa, K. Development of Radiogallium-Labeled Peptides for Platelet-Derived Growth Factor Receptor β (PDGFRβ) Imaging: Influence of Different Linkers. Molecules 2020, 26, 41. [Google Scholar] [CrossRef]
- Doubrovin, M.; Kochetkova, T.; Santos, E.; Veach, D.R.; Smith-Jones, P.; Pillarsetty, N.; Balatoni, J.; Bornmann, W.; Gelovani, J.; Larson, S.M. (124)I-iodopyridopyrimidinone for PET of Abl kinase-expressing tumors in vivo. J. Nucl. Med. 2010, 51, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Benezra, M.; Hambardzumyan, D.; Penate-Medina, O.; Veach, D.R.; Pillarsetty, N.; Smith-Jones, P.; Phillips, E.; Ozawa, T.; Zanzonico, P.B.; Longo, V.; et al. Fluorine-labeled dasatinib nanoformulations as targeted molecular imaging probes in a PDGFB-driven murine glioblastoma model. Neoplasia 2012, 14, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Maxwell, D.S.; Sun, D.; Bhanu Prasad, B.A.; Pal, A.; Wang, S.; Balatoni, J.; Ghosh, P.; Lim, S.T.; Volgin, A.; et al. Imatinib analogs as potential agents for PET imaging of Bcr-Abl and c-KIT expression at a kinase level. Bioorg. Med. Chem. 2014, 22, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Caballero, J.; Muñoz, C.; Alzate-Morales, J.H.; Cunha, S.; Gano, L.; Bergmann, R.; Steinbach, J.; Kniess, T. Synthesis, in silico, in vitro, and in vivo investigation of 5-[¹¹C]methoxy-substituted sunitinib, a tyrosine kinase inhibitor of VEGFR-2. Eur. J. Med. Chem. 2012, 58, 272–280. [Google Scholar] [CrossRef]
- Slobbe, P.; Poot, A.J.; Haumann, R.; Schuit, R.C.; Windhorst, A.D.; van Dongen, G.A. Two anti-angiogenic TKI-PET tracers, [(11)C]axitinib and [(11)C]nintedanib: Radiosynthesis, in vivo metabolism and initial biodistribution studies in rodents. Nucl. Med. Biol. 2016, 43, 612–624. [Google Scholar] [CrossRef]
- Poot, A.J.; van der Wildt, B.; Stigter-van Walsum, M.; Rongen, M.; Schuit, R.C.; Hendrikse, N.H.; Eriksson, J.; van Dongen, G.A.; Windhorst, A.D. [¹¹C]Sorafenib: Radiosynthesis and preclinical evaluation in tumor-bearing mice of a new TKI-PET tracer. Nucl. Med. Biol. 2013, 40, 488–497. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Pascual, A.; Siebzehnrubl, F.A. Fibroblast Growth Factor Receptor Functions in Glioblastoma. Cells 2019, 8, 715. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Pascual, A.; Lathia, J.D.; Siebzehnrubl, F.A. ADAMDEC1 and FGF2/FGFR1 signaling constitute a positive feedback loop to maintain GBM cancer stem cells. Mol. Cell Oncol. 2020, 7, 1684787. [Google Scholar] [CrossRef]
- Gouazé-Andersson, V.; Delmas, C.; Taurand, M.; Martinez-Gala, J.; Evrard, S.; Mazoyer, S.; Toulas, C.; Cohen-Jonathan-Moyal, E. FGFR1 Induces Glioblastoma Radioresistance through the PLCγ/Hif1α Pathway. Cancer Res. 2016, 76, 3036–3044. [Google Scholar] [CrossRef] [Green Version]
- Kowalski-Chauvel, A.; Gouaze-Andersson, V.; Baricault, L.; Martin, E.; Delmas, C.; Toulas, C.; Cohen-Jonathan-Moyal, E.; Seva, C. Alpha6-Integrin Regulates FGFR1 Expression through the ZEB1/YAP1 Transcription Complex in Glioblastoma Stem Cells Resulting in Enhanced Proliferation and Stemness. Cancers 2019, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Lasorella, A.; Sanson, M.; Iavarone, A. FGFR-TACC gene fusions in human glioma. Neuro Oncol. 2017, 19, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Bahleda, R.; Dienstmann, R.; Infante, J.R.; Mita, A.; Italiano, A.; Calvo, E.; Moreno, V.; Adamo, B.; Gazzah, A.; et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2015, 33, 3401–3408. [Google Scholar] [CrossRef]
- Nishina, T.; Takahashi, S.; Iwasawa, R.; Noguchi, H.; Aoki, M.; Doi, T. Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Investig. New Drugs 2018, 36, 424–434. [Google Scholar] [CrossRef]
- Bahleda, R.; Meric-Bernstam, F.; Goyal, L.; Tran, B.; He, Y.; Yamamiya, I.; Benhadji, K.A.; Matos, I.; Arkenau, H.T. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann. Oncol. 2020, 31, 1405–1412. [Google Scholar] [CrossRef]
- Nogova, L.; Sequist, L.V.; Perez Garcia, J.M.; Andre, F.; Delord, J.P.; Hidalgo, M.; Schellens, J.H.; Cassier, P.A.; Camidge, D.R.; Schuler, M.; et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients with Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J. Clin. Oncol. 2017, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bahleda, R.; Italiano, A.; Hierro, C.; Mita, A.; Cervantes, A.; Chan, N.; Awad, M.; Calvo, E.; Moreno, V.; Govindan, R.; et al. Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors. Clin. Cancer Res. 2019, 25, 4888–4897. [Google Scholar] [CrossRef] [PubMed]
- Babina, I.S.; Turner, N.C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 2017, 17, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.H.; Pan, L.H.; Wong, P.T.; Chen, W.H.; Yang, Y.Q.; Wang, H.; Xiang, J.J.; Xu, M. (125)I-labeled anti-bFGF monoclonal antibody inhibits growth of hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 5033–5041. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tai, W.T.; Wu, S.Y.; Shih, C.T.; Chen, M.H.; Tsai, M.H.; Kuo, C.W.; Shiau, C.W.; Hung, M.H.; Chen, K.F. Dovitinib Acts as a Novel Radiosensitizer in Hepatocellular Carcinoma by Targeting SHP-1/STAT3 Signaling. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 761–771. [Google Scholar] [CrossRef]
- Janes, P.W.; Vail, M.E.; Gan, H.K.; Scott, A.M. Antibody Targeting of Eph Receptors in Cancer. Pharmaceuticals 2020, 13, 88. [Google Scholar] [CrossRef]
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 465–487. [Google Scholar] [CrossRef] [Green Version]
- Anderton, M.; van der Meulen, E.; Blumenthal, M.J.; Schäfer, G. The Role of the Eph Receptor Family in Tumorigenesis. Cancers 2021, 13, 206. [Google Scholar] [CrossRef]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Zhao, W.; Yuan, S.; Yu, J.; Zhao, S.; Ma, L.; Zhang, D.; Hu, X. A novel molecular agent for glioma angiogenesis imaging. Nucl. Med. Commun. 2017, 38, 919–926. [Google Scholar] [CrossRef]
- Ferluga, S.; Tomé, C.M.; Herpai, D.M.; D’Agostino, R.; Debinski, W. Simultaneous targeting of Eph receptors in glioblastoma. Oncotarget 2016, 7, 59860–59876. [Google Scholar] [CrossRef] [Green Version]
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K.L.; Huse, J.T.; Cajola, L.; Zanetti, N.; DiMeco, F.; et al. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offenhäuser, C.; Al-Ejeh, F.; Puttick, S.; Ensbey, K.S.; Bruce, Z.C.; Jamieson, P.R.; Smith, F.M.; Stringer, B.W.; Carrington, B.; Fuchs, A.V.; et al. EphA3 Pay-Loaded Antibody Therapeutics for the Treatment of Glioblastoma. Cancers 2018, 10, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.D.; Rath, P.; Lal, B.; Richard, J.P.; Li, Y.; Goodwin, C.R.; Laterra, J.; Xia, S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 2012, 31, 5132–5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, M.; Niska, J.A.; Miyamori, H.; McDonough, W.S.; Wu, J.; Sato, H.; Berens, M.E. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004, 64, 3179–3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swords, R.T.; Greenberg, P.L.; Wei, A.H.; Durrant, S.; Advani, A.S.; Hertzberg, M.S.; Jonas, B.A.; Lewis, I.D.; Rivera, G.; Gratzinger, D.; et al. KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3, in patients with advanced hematologic malignancies: Results from a phase 1 study. Leuk. Res. 2016, 50, 123–131. [Google Scholar] [CrossRef]
- Hui, G.; Lawrence, C.; Po, I.; Zarnie, L.; Eddie, L.; Christian, W.; Alex, M.; Uwe, A.; Nicole, C.; Kristen, R.; et al. Phase I safety and bioimaging trial of KB004 (ifabotuzumab) in patients with glioblastoma. J. Nucl. Med. 2020, 61, 1562. [Google Scholar]
- Okada, H.; Kalinski, P.; Ueda, R.; Hoji, A.; Kohanbash, G.; Donegan, T.E.; Mintz, A.H.; Engh, J.A.; Bartlett, D.L.; Brown, C.K.; et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 2011, 29, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Shitara, K.; Satoh, T.; Iwasa, S.; Yamaguchi, K.; Muro, K.; Komatsu, Y.; Nishina, T.; Esaki, T.; Hasegawa, J.; Kakurai, Y.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: A first-in-human phase I dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 219. [Google Scholar] [CrossRef]
- Gravina, G.L.; Mancini, A.; Colapietro, A.; Delle Monache, S.; Sferra, R.; Vitale, F.; Cristiano, L.; Martellucci, S.; Marampon, F.; Mattei, V.; et al. The Small Molecule Ephrin Receptor Inhibitor, GLPG1790, Reduces Renewal Capabilities of Cancer Stem Cells, Showing Anti-Tumour Efficacy on Preclinical Glioblastoma Models. Cancers 2019, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tang, S.; Yu, Y.; Lv, Y.; Wang, A.; Yan, X.; Li, N.; Sha, C.; Sun, K.; Li, Y. Intranasal Delivery of Temozolomide-Conjugated Gold Nanoparticles Functionalized with Anti-EphA3 for Glioblastoma Targeting. Mol. Pharm. 2021, 18, 915–927. [Google Scholar] [CrossRef]
- Bhatia, S.; Bukkapatnam, S.; Van Court, B.; Phan, A.; Oweida, A.; Gadwa, J.; Mueller, A.C.; Piper, M.; Darragh, L.; Nguyen, D.; et al. The effects of ephrinB2 signaling on proliferation and invasion in glioblastoma multiforme. Mol. Carcinog. 2020, 59, 1064–1075. [Google Scholar] [CrossRef]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Adams, J.; Singh, M.; Hu, A.; Gorelik, M.; Subapanditha, M.K.; Savage, N.; Yang, J.; et al. Cotargeting Ephrin Receptor Tyrosine Kinases A2 and A3 in Cancer Stem Cells Reduces Growth of Recurrent Glioblastoma. Cancer Res. 2018, 78, 5023–5037. [Google Scholar] [CrossRef] [Green Version]
- Andrews, D.W.; Resnicoff, M.; Flanders, A.E.; Kenyon, L.; Curtis, M.; Merli, G.; Baserga, R.; Iliakis, G.; Aiken, R.D. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J. Clin. Oncol. 2001, 19, 2189–2200. [Google Scholar] [CrossRef]
- Harshyne, L.A.; Hooper, K.M.; Andrews, E.G.; Nasca, B.J.; Kenyon, L.C.; Andrews, D.W.; Hooper, D.C. Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol. Immunother. 2015, 64, 299–309. [Google Scholar] [CrossRef]
- Aiken, R.; Axelson, M.; Harmenberg, J.; Klockare, M.; Larsson, O.; Wassberg, C. Phase I clinical trial of AXL1717 for treatment of relapsed malignant astrocytomas: Analysis of dose and response. Oncotarget 2017, 8, 81501–81510. [Google Scholar] [CrossRef] [Green Version]
- Neuber, C.; Belter, B.; Mamat, C.; Pietzsch, J. Radiopharmacologist’s and Radiochemist’s View on Targeting the Eph/Ephrin Receptor Tyrosine Kinase System. ACS Omega 2020, 5, 16318–16331. [Google Scholar] [CrossRef]
- Cai, W.; Ebrahimnejad, A.; Chen, K.; Cao, Q.; Li, Z.B.; Tice, D.A.; Chen, X. Quantitative radioimmunoPET imaging of EphA2 in tumor-bearing mice. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2024–2036. [Google Scholar] [CrossRef]
- Day, B.W.; Stringer, B.W.; Al-Ejeh, F.; Ting, M.J.; Wilson, J.; Ensbey, K.S.; Jamieson, P.R.; Bruce, Z.C.; Lim, Y.C.; Offenhäuser, C.; et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell 2013, 23, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Charmsaz, S.; Al-Ejeh, F.; Yeadon, T.M.; Miller, K.J.; Smith, F.M.; Stringer, B.W.; Moore, A.S.; Lee, F.T.; Cooper, L.T.; Stylianou, C.; et al. EphA3 as a target for antibody immunotherapy in acute lymphoblastic leukemia. Leukemia 2017, 31, 1779–1787. [Google Scholar] [CrossRef] [Green Version]
- Puttick, S.; Stringer, B.W.; Day, B.W.; Bruce, Z.C.; Ensbey, K.S.; Mardon, K.; Cowin, G.J.; Thurecht, K.J.; Whittaker, A.K.; Fay, M.; et al. EphA2 as a Diagnostic Imaging Target in Glioblastoma: A Positron Emission Tomography/Magnetic Resonance Imaging Study. Mol. Imaging 2015, 14, 385–399. [Google Scholar] [CrossRef]
- Burvenich, I.J.; Parakh, S.; Gan, H.K.; Lee, F.T.; Guo, N.; Rigopoulos, A.; Lee, S.T.; Gong, S.; O’Keefe, G.J.; Tochon-Danguy, H.; et al. Molecular Imaging and Quantitation of EphA2 Expression in Xenograft Models with 89Zr-DS-8895a. J. Nucl. Med. 2016, 57, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Xiong, C.; Lu, W.; Zhang, R.; Zhou, M.; Huang, Q.; Weinberg, J.; Li, C. Dual-modality micro-positron emission tomography/computed tomography and near-infrared fluorescence imaging of EphB4 in orthotopic glioblastoma xenograft models. Mol. Imaging Biol. 2014, 16, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Mamat, C.; Mosch, B.; Neuber, C.; Köckerling, M.; Bergmann, R.; Pietzsch, J. Fluorine-18 radiolabeling and radiopharmacological characterization of a benzodioxolylpyrimidine-based radiotracer targeting the receptor tyrosine kinase EphB4. ChemMedChem 2012, 7, 1991–2003. [Google Scholar] [CrossRef]
- Ebert, K.; Wiemer, J.; Caballero, J.; Köckerling, M.; Steinbach, J.; Pietzsch, J.; Mamat, C. Development of indazolylpyrimidine derivatives as high-affine EphB4 receptor ligands and potential PET radiotracers. Bioorg. Med. Chem. 2015, 23, 6025–6035. [Google Scholar] [CrossRef]
- Xiong, C.; Huang, M.; Zhang, R.; Song, S.; Lu, W.; Flores, L., 2nd; Gelovani, J.; Li, C. In vivo small-animal PET/CT of EphB4 receptors using 64Cu-labeled peptide. J. Nucl. Med. 2011, 52, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Noberini, R.; Lamberto, I.; Pasquale, E.B. Targeting Eph receptors with peptides and small molecules: Progress and challenges. Semin. Cell. Dev. Biol. 2012, 23, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Pretze, M.; Neuber, C.; Kinski, E.; Belter, B.; Köckerling, M.; Caflisch, A.; Steinbach, J.; Pietzsch, J.; Mamat, C. Synthesis, radiolabelling and initial biological characterisation of (18)F-labelled xanthine derivatives for PET imaging of Eph receptors. Org. Biomol. Chem. 2020, 18, 3104–3116. [Google Scholar] [CrossRef]
- Osher, E.; Macaulay, V.M. Therapeutic Targeting of the IGF Axis. Cells 2019, 8, 895. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Pourpak, A.; Morris, S.W. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J. Med. Chem. 2009, 52, 4981–5004. [Google Scholar] [CrossRef] [Green Version]
- Samani, A.A.; Yakar, S.; LeRoith, D.; Brodt, P. The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr. Rev. 2007, 28, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Schlenska-Lange, A.; Knüpfer, H.; Lange, T.J.; Kiess, W.; Knüpfer, M. Cell proliferation and migration in glioblastoma multiforme cell lines are influenced by insulin-like growth factor I in vitro. Anticancer Res. 2008, 28, 1055–1060. [Google Scholar] [PubMed]
- Zamykal, M.; Martens, T.; Matschke, J.; Günther, H.S.; Kathagen, A.; Schulte, A.; Peters, R.; Westphal, M.; Lamszus, K. Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms. Neuro Oncol. 2015, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Loeffler, J.S.; Dyson, N.J. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002, 62, 200–207. [Google Scholar]
- Mellinghoff, I.K.; Wang, M.Y.; Vivanco, I.; Haas-Kogan, D.A.; Zhu, S.; Dia, E.Q.; Lu, K.V.; Yoshimoto, K.; Huang, J.H.; Chute, D.J.; et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 2005, 353, 2012–2024. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.D.; Goss, P.E. The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist 2008, 13, 16–24. [Google Scholar] [CrossRef]
- Trojan, J.; Cloix, J.F.; Ardourel, M.Y.; Chatel, M.; Anthony, D.D. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience 2007, 145, 795–811. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, F.; Ma, P.; Hui, H.; Pei, S.; Chen, M.; Wang, Z.; Zhou, W.; Jin, B. GSK1838705A, an IGF-1R inhibitor, inhibits glioma cell proliferation and suppresses tumor growth in vivo. Mol. Med. Rep. 2015, 12, 5641–5646. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhao, X.; Li, X.; Ping, G.; Pei, S.; Chen, M.; Wang, Z.; Zhou, W.; Jin, B. PQ401, an IGF-1R inhibitor, induces apoptosis and inhibits growth, proliferation and migration of glioma cells. J. Chemother. 2016, 28, 44–49. [Google Scholar] [CrossRef]
- Premkumar, D.R.; Jane, E.P.; Pollack, I.F. Co-administration of NVP-AEW541 and dasatinib induces mitochondrial-mediated apoptosis through Bax activation in malignant human glioma cell lines. Int. J. Oncol. 2010, 37, 633–643. [Google Scholar] [CrossRef]
- Yin, S.; Girnita, A.; Strömberg, T.; Khan, Z.; Andersson, S.; Zheng, H.; Ericsson, C.; Axelson, M.; Nistér, M.; Larsson, O.; et al. Targeting the insulin-like growth factor-1 receptor by picropodophyllin as a treatment option for glioblastoma. Neuro Oncol. 2010, 12, 19–27. [Google Scholar] [CrossRef]
- Fuentes-Baile, M.; Ventero, M.P.; Encinar, J.A.; García-Morales, P.; Poveda-Deltell, M.; Pérez-Valenciano, E.; Barberá, V.M.; Gallego-Plazas, J.; Rodríguez-Lescure, Á.; Martín-Nieto, J.; et al. Differential Effects of IGF-1R Small Molecule Tyrosine Kinase Inhibitors BMS-754807 and OSI-906 on Human Cancer Cell Lines. Cancers 2020, 12, 3717. [Google Scholar] [CrossRef]
- Baserga, R. The decline and fall of the IGF-I receptor. J. Cell Physiol. 2013, 228, 675–679. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Spigel, D.R.; Chen, D.; Steins, M.B.; Engelman, J.A.; Schneider, C.P.; Novello, S.; Eberhardt, W.E.; Crino, L.; Habben, K.; et al. Randomized phase II study of erlotinib in combination with placebo or R1507, a monoclonal antibody to insulin-like growth factor-1 receptor, for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 4574–4580. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.S.; Piulats, J.M.; Pandha, H.S.; Petrylak, D.P.; Saad, F.; Aparicio, L.M.; Sandhu, S.K.; Fong, P.; Gillessen, S.; Hudes, G.R.; et al. Phase II randomized study of figitumumab plus docetaxel and docetaxel alone with crossover for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2014, 20, 1925–1934. [Google Scholar] [CrossRef] [Green Version]
- Philip, P.A.; Goldman, B.; Ramanathan, R.K.; Lenz, H.J.; Lowy, A.M.; Whitehead, R.P.; Wakatsuki, T.; Iqbal, S.; Gaur, R.; Benedetti, J.K.; et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: Phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 2014, 120, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Moran, T.; Felip, E.; Keedy, V.; Borghaei, H.; Shepherd, F.A.; Insa, A.; Brown, H.; Fitzgerald, T.; Sathyanarayanan, S.; Reilly, J.F.; et al. Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with Non-small-cell lung cancer: A phase I/II randomized trial. Exp. Hematol. Oncol. 2014, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Houghton, P.J.; Morton, C.L.; Gorlick, R.; Kolb, E.A.; Keir, S.T.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Wu, J.; Smith, M.A. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr. Blood Cancer 2010, 54, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Higano, C.S.; Berlin, J.; Gordon, M.; LoRusso, P.; Tang, S.; Dontabhaktuni, A.; Schwartz, J.D.; Cosaert, J.; Mehnert, J.M. Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Investig. New Drugs 2015, 33, 450–462. [Google Scholar] [CrossRef]
- Osuka, S.; Sampetrean, O.; Shimizu, T.; Saga, I.; Onishi, N.; Sugihara, E.; Okubo, J.; Fujita, S.; Takano, S.; Matsumura, A.; et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells 2013, 31, 627–640. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Shen, B. Molecular Imaging of IGF-1R in Cancer. Mol. Imaging 2017, 16, 1536012117736648. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, B.; McLarty, K.; Kersemans, V.; Reilly, R.M. The level of insulin growth factor-1 receptor expression is directly correlated with the tumor uptake of (111)In-IGF-1(E3R) in vivo and the clonogenic survival of breast cancer cells exposed in vitro to trastuzumab (Herceptin). Nucl. Med. Biol. 2008, 35, 645–653. [Google Scholar] [CrossRef]
- Fleuren, E.D.; Versleijen-Jonkers, Y.M.; van de Luijtgaarden, A.C.; Molkenboer-Kuenen, J.D.; Heskamp, S.; Roeffen, M.H.; van Laarhoven, H.W.; Houghton, P.J.; Oyen, W.J.; Boerman, O.C.; et al. Predicting IGF-1R therapy response in bone sarcomas: Immuno-SPECT imaging with radiolabeled R1507. Clin. Cancer Res. 2011, 17, 7693–7703. [Google Scholar] [CrossRef] [Green Version]
- Heskamp, S.; van Laarhoven, H.W.; Molkenboer-Kuenen, J.D.; Bouwman, W.H.; van der Graaf, W.T.; Oyen, W.J.; Boerman, O.C. Optimization of IGF-1R SPECT/CT imaging using 111In-labeled F(ab′)2 and Fab fragments of the monoclonal antibody R1507. Mol. Pharm. 2012, 9, 2314–2321. [Google Scholar] [CrossRef]
- Prabhakaran, J.; Dewey, S.L.; McClure, R.; Simpson, N.R.; Tantawy, M.N.; Mann, J.J.; Pham, W.; Kumar, J.S.D. In vivo evaluation of IGF1R/IR PET ligand [(18)F]BMS-754807 in rodents. Bioorg. Med. Chem. Lett. 2017, 27, 941–943. [Google Scholar] [CrossRef] [Green Version]
- Majo, V.J.; Arango, V.; Simpson, N.R.; Prabhakaran, J.; Kassir, S.A.; Underwood, M.D.; Bakalian, M.; Canoll, P.; John Mann, J.; Dileep Kumar, J.S. Synthesis and in vitro evaluation of [18F]BMS-754807: A potential PET ligand for IGF-1R. Bioorg. Med. Chem. Lett. 2013, 23, 4191–4194. [Google Scholar] [CrossRef] [Green Version]
- Solingapuram Sai, K.K.; Prabhakaran, J.; Sattiraju, A.; Mann, J.J.; Mintz, A.; Kumar, J.S.D. Radiosynthesis and evaluation of IGF1R PET ligand [(11)C]GSK1838705A. Bioorg. Med. Chem. Lett. 2017, 27, 2895–2897. [Google Scholar] [CrossRef]
- Solomon, V.R.; Alizadeh, E.; Bernhard, W.; Hartimath, S.V.; Hill, W.; Chekol, R.; Barreto, K.M.; Geyer, C.R.; Fonge, H. (111)In- and (225)Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer. Mol. Pharm. 2019, 16, 4807–4816. [Google Scholar] [CrossRef]
- Solomon, V.R.; Alizadeh, E.; Bernhard, W.; Makhlouf, A.; Hartimath, S.V.; Hill, W.; El-Sayed, A.; Barreto, K.; Geyer, C.R.; Fonge, H. Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer. Sci. Rep. 2020, 10, 18549. [Google Scholar] [CrossRef]
- Orlova, A.; Hofström, C.; Strand, J.; Varasteh, Z.; Sandstrom, M.; Andersson, K.; Tolmachev, V.; Gräslund, T. [99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a new Affibody conjugate for visualization of insulin-like growth factor-1 receptor expression in malignant tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Mitran, B.; Altai, M.; Hofström, C.; Honarvar, H.; Sandström, M.; Orlova, A.; Tolmachev, V.; Gräslund, T. Evaluation of 99mTc-Z IGF1R:4551-GGGC affibody molecule, a new probe for imaging of insulin-like growth factor type 1 receptor expression. Amino Acids 2015, 47, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolmachev, V.; Malmberg, J.; Hofström, C.; Abrahmsén, L.; Bergman, T.; Sjöberg, A.; Sandström, M.; Gräslund, T.; Orlova, A. Imaging of insulinlike growth factor type 1 receptor in prostate cancer xenografts using the affibody molecule 111In-DOTA-ZIGF1R:4551. J. Nucl. Med. 2012, 53, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Cheng, K.; Liu, Y.; Hu, X.; Meng, S.; Cheng, Z. PET imaging of insulin-like growth factor type 1 receptor expression with a 64Cu-labeled Affibody molecule. Amino Acids 2015, 47, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Day, E.K.; Sosale, N.G.; Xiao, A.; Zhong, Q.; Purow, B.; Lazzara, M.J. Glioblastoma Cell Resistance to EGFR and MET Inhibition Can Be Overcome via Blockade of FGFR-SPRY2 Bypass Signaling. Cell Rep. 2020, 30, 3383–3396.e7. [Google Scholar] [CrossRef] [Green Version]
- Ou, A.; Ott, M.; Fang, D.; Heimberger, A.B. The Role and Therapeutic Targeting of JAK/STAT Signaling in Glioblastoma. Cancers 2021, 13, 437. [Google Scholar] [CrossRef]
- Zhao, H.F.; Wang, J.; Shao, W.; Wu, C.P.; Chen, Z.P.; To, S.T.; Li, W.P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef] [Green Version]
- Cloughesy, T.F.; Drappatz, J.; de Groot, J.; Prados, M.D.; Reardon, D.A.; Schiff, D.; Chamberlain, M.; Mikkelsen, T.; Desjardins, A.; Ping, J.; et al. Phase II study of cabozantinib in patients with progressive glioblastoma: Subset analysis of patients with prior antiangiogenic therapy. Neuro Oncol. 2018, 20, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Lassman, A.B.; Pugh, S.L.; Gilbert, M.R.; Aldape, K.D.; Geinoz, S.; Beumer, J.H.; Christner, S.M.; Komaki, R.; DeAngelis, L.M.; Gaur, R.; et al. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol. 2015, 17, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.Y.; Drappatz, J.; de Groot, J.; Prados, M.D.; Reardon, D.A.; Schiff, D.; Chamberlain, M.; Mikkelsen, T.; Desjardins, A.; Holland, J.; et al. Phase II study of cabozantinib in patients with progressive glioblastoma: Subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018, 20, 249–258. [Google Scholar] [CrossRef]
- Franceschi, E.; Stupp, R.; van den Bent, M.J.; van Herpen, C.; Laigle Donadey, F.; Gorlia, T.; Hegi, M.; Lhermitte, B.; Strauss, L.C.; Allgeier, A.; et al. EORTC 26083 phase I/II trial of dasatinib in combination with CCNU in patients with recurrent glioblastoma. Neuro Oncol. 2012, 14, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Kreisl, T.N.; McNeill, K.A.; Sul, J.; Iwamoto, F.M.; Shih, J.; Fine, H.A. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012, 14, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Vredenburgh, J.J.; Desjardins, A.; Peters, K.B.; Sathornsumetee, S.; Threatt, S.; Sampson, J.H.; Herndon, J.E., 2nd; Coan, A.; McSherry, F.; et al. Phase 1 trial of dasatinib plus erlotinib in adults with recurrent malignant glioma. J. Neurooncol. 2012, 108, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Miklja, Z.; Yadav, V.N.; Cartaxo, R.T.; Siada, R.; Thomas, C.C.; Cummings, J.R.; Mullan, B.; Stallard, S.; Paul, A.; Bruzek, A.K.; et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J. Clin. Investig. 2020, 130, 5313–5325. [Google Scholar] [CrossRef]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Hamed, H.A.; Tavallai, S.; Grant, S.; Poklepovic, A.; Dent, P. Sorafenib/regorafenib and lapatinib interact to kill CNS tumor cells. J. Cell Physiol. 2015, 230, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef]
- Subbiah, V.; Khawaja, M.R.; Hong, D.S.; Amini, B.; Yungfang, J.; Liu, H.; Johnson, A.; Schrock, A.B.; Ali, S.M.; Sun, J.X.; et al. First-in-human trial of multikinase VEGF inhibitor regorafenib and anti-EGFR antibody cetuximab in advanced cancer patients. JCI Insight 2017, 2, e90380. [Google Scholar] [CrossRef]
- Razis, E.; Selviaridis, P.; Labropoulos, S.; Norris, J.L.; Zhu, M.J.; Song, D.D.; Kalebic, T.; Torrens, M.; Kalogera-Fountzila, A.; Karkavelas, G.; et al. Phase II study of neoadjuvant imatinib in glioblastoma: Evaluation of clinical and molecular effects of the treatment. Clin. Cancer Res. 2009, 15, 6258–6266. [Google Scholar] [CrossRef] [Green Version]
- Sautter, L.; Hofheinz, R.; Tuettenberg, J.; Grimm, M.; Vajkoczy, P.; Groden, C.; Schmieder, K.; Hochhaus, A.; Wenz, F.; Giordano, F.A. Open-Label Phase II Evaluation of Imatinib in Primary Inoperable or Incompletely Resected and Recurrent Glioblastoma. Oncology 2020, 98, 16–22. [Google Scholar] [CrossRef]
- Wen, P.Y.; Yung, W.K.; Lamborn, K.R.; Dahia, P.L.; Wang, Y.; Peng, B.; Abrey, L.E.; Raizer, J.; Cloughesy, T.F.; Fink, K.; et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin. Cancer Res. 2006, 12, 4899–4907. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Egorin, M.J.; Quinn, J.A.; Rich, J.N.; Gururangan, S.; Vredenburgh, J.J.; Desjardins, A.; Sathornsumetee, S.; Provenzale, J.M.; Herndon, J.E., 2nd; et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J. Clin. Oncol. 2005, 23, 9359–9368. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Quinn, J.A.; Vredenburgh, J.J.; Sathornsumetee, S.; Friedman, A.H.; Herndon, J.E.; McLendon, R.E.; Provenzale, J.M.; Rich, J.N.; Sampson, J.H.; et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J. Neurooncol. 2007, 83, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, J.; Guan, R.; Zhu, Z.; Yuan, X. Tyrphostin AG 1296 induces glioblastoma cell apoptosis in vitro and in vivo. Oncol. Lett. 2015, 10, 3429–3433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiessen, B.; Stewart, C.; Tsao, M.; Kamel-Reid, S.; Schaiquevich, P.; Mason, W.; Easaw, J.; Belanger, K.; Forsyth, P.; McIntosh, L.; et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: Clinical outcomes, pharmacokinetics and molecular correlation. Cancer Chemother. Pharmacol. 2010, 65, 353–361. [Google Scholar] [CrossRef]
- Yu, A.; Faiq, N.; Green, S.; Lai, A.; Green, R.; Hu, J.; Cloughesy, T.F.; Mellinghoff, I.; Nghiemphu, P.L. Report of safety of pulse dosing of lapatinib with temozolomide and radiation therapy for newly-diagnosed glioblastoma in a pilot phase II study. J. Neurooncol. 2017, 134, 357–362. [Google Scholar] [CrossRef]
- Karavasilis, V.; Kotoula, V.; Pentheroudakis, G.; Televantou, D.; Lambaki, S.; Chrisafi, S.; Bobos, M.; Fountzilas, G. A phase I study of temozolomide and lapatinib combination in patients with recurrent high-grade gliomas. J. Neurol. 2013, 260, 1469–1480. [Google Scholar] [CrossRef]
- Reardon, D.A.; Groves, M.D.; Wen, P.Y.; Nabors, L.; Mikkelsen, T.; Rosenfeld, S.; Raizer, J.; Barriuso, J.; McLendon, R.E.; Suttle, A.B.; et al. A phase I/II trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin. Cancer Res. 2013, 19, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Deeks, E.D. Neratinib: First Global Approval. Drugs 2017, 77, 1695–1704. [Google Scholar] [CrossRef]
- Alexander, B.M.; Trippa, L.; Gaffey, S.; Arrillaga-Romany, I.C.; Lee, E.Q.; Rinne, M.L.; Ahluwalia, M.S.; Colman, H.; Fell, G.; Galanis, E.; et al. Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT): A Bayesian Adaptive Platform Trial to Develop Precision Medicines for Patients with Glioblastoma. JCO Precis. Oncol. 2019, 3, po.18.00071. [Google Scholar] [CrossRef]
- Schiff, D.; Jaeckle, K.A.; Anderson, S.K.; Galanis, E.; Giannini, C.; Buckner, J.C.; Stella, P.; Flynn, P.J.; Erickson, B.J.; Schwerkoske, J.F.; et al. Phase 1/2 trial of temsirolimus and sorafenib in the treatment of patients with recurrent glioblastoma: North Central Cancer Treatment Group Study/Alliance N0572. Cancer 2018, 124, 1455–1463. [Google Scholar] [CrossRef]
- Peereboom, D.M.; Ahluwalia, M.S.; Ye, X.; Supko, J.G.; Hilderbrand, S.L.; Phuphanich, S.; Nabors, L.B.; Rosenfeld, M.R.; Mikkelsen, T.; Grossman, S.A. NABTT 0502: A phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013, 15, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Reardon, D.A.; Vredenburgh, J.J.; Desjardins, A.; Peters, K.; Gururangan, S.; Sampson, J.H.; Marcello, J.; Herndon, J.E., 2nd; McLendon, R.E.; Janney, D.; et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: Results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J. Neurooncol. 2011, 101, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Hottinger, A.F.; Ben Aissa, A.; Espeli, V.; Squiban, D.; Dunkel, N.; Vargas, M.I.; Hundsberger, T.; Mach, N.; Schaller, K.; Weber, D.C.; et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br. J. Cancer 2014, 110, 2655–2661. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Ervin, T.; Friedman, E.; Priego, V.; Murphy, P.B.; Clark, B.L.; Lamar, R.E. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer 2010, 116, 3663–3669. [Google Scholar] [CrossRef]
- Nghiemphu, P.L.; Ebiana, V.A.; Wen, P.; Gilbert, M.; Abrey, L.E.; Lieberman, F.; DeAngelis, L.M.; Robins, H.I.; Yung, W.K.A.; Chang, S.; et al. Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05-02. J. Neurooncol. 2018, 136, 79–86. [Google Scholar] [CrossRef]
- Kreisl, T.N.; Smith, P.; Sul, J.; Salgado, C.; Iwamoto, F.M.; Shih, J.H.; Fine, H.A. Continuous daily sunitinib for recurrent glioblastoma. J. Neurooncol. 2013, 111, 41–48. [Google Scholar] [CrossRef]
- Balaña, C.; Gil, M.J.; Perez, P.; Reynes, G.; Gallego, O.; Ribalta, T.; Capellades, J.; Gonzalez, S.; Verger, E. Sunitinib administered prior to radiotherapy in patients with non-resectable glioblastoma: Results of a phase II study. Target. Oncol. 2014, 9, 321–329. [Google Scholar] [CrossRef]
- Reardon, D.A.; Vredenburgh, J.J.; Coan, A.; Desjardins, A.; Peters, K.B.; Gururangan, S.; Sathornsumetee, S.; Rich, J.N.; Herndon, J.E.; Friedman, H.S. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J. Neurooncol. 2011, 105, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Pan, E.; Yu, D.; Yue, B.; Potthast, L.; Chowdhary, S.; Smith, P.; Chamberlain, M. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J. Neurooncol. 2012, 110, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Hutterer, M.; Nowosielski, M.; Haybaeck, J.; Embacher, S.; Stockhammer, F.; Gotwald, T.; Holzner, B.; Capper, D.; Preusser, M.; Marosi, C.; et al. A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01-07). Neuro Oncol. 2014, 16, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Muhic, A.; Poulsen, H.S.; Sorensen, M.; Grunnet, K.; Lassen, U. Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J. Neurooncol. 2013, 111, 205–212. [Google Scholar] [CrossRef]
- Norden, A.D.; Schiff, D.; Ahluwalia, M.S.; Lesser, G.J.; Nayak, L.; Lee, E.Q.; Rinne, M.L.; Muzikansky, A.; Dietrich, J.; Purow, B.; et al. Phase II trial of triple tyrosine kinase receptor inhibitor nintedanib in recurrent high-grade gliomas. J. Neurooncol. 2015, 121, 297–302. [Google Scholar] [CrossRef]
- Reardon, D.A.; Pan, E.; Fan, J.; Mink, J.; Barboriak, D.P.; Vredenburgh, J.J.; Desjardins, A.; Peters, K.; O’Brien, J.P.; Wen, P.Y. 417PD—A Phase 2 Trial of the Multitargeted Kinase Inhibitor Lenvatinib (E7080) in Patients (PTS) with Recurrent Glioblastoma (GBM) And Disease Progression Following Prior Bevacizumab Treatment. Ann. Oncol. 2012, 23, ix146. [Google Scholar] [CrossRef]
- Lee, E.Q.; Muzikansky, A.; Duda, D.G.; Gaffey, S.; Dietrich, J.; Nayak, L.; Chukwueke, U.N.; Beroukhim, R.; Doherty, L.; Laub, C.K.; et al. Phase II trial of ponatinib in patients with bevacizumab-refractory glioblastoma. Cancer Med. 2019, 8, 5988–5994. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, D.; Chen, J.; Chen, H.; Fan, R.; Gao, Y.; Tao, R.; Zhang, H. Targeted Therapy with Anlotinib for a Patient with an Oncogenic FGFR3-TACC3 Fusion and Recurrent Glioblastoma. Oncologist 2021, 26, 173–177. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Liu, F.; Song, M.; Hou, Y.; Liang, N. Targeted therapy with anlotinib for patient with recurrent glioblastoma: A case report and literature review. Medicine 2019, 98, e15749. [Google Scholar] [CrossRef]
- Das, M.; Padda, S.K.; Frymoyer, A.; Molina, J.; Adjei, A.; Lensing, J.L.; Miles, D.; Sikic, B.I.; Wakelee, H.A. A safety, tolerability, and pharmacokinetic analysis of two phase I studies of multitargeted small molecule tyrosine kinase inhibitor XL647 with an intermittent and continuous dosing schedule in patients with advanced solid malignancies. Cancer Chemother. Pharmacol. 2018, 82, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kizilbash, S.H.; Gupta, S.K.; Parrish, K.E.; Laramy, J.K.; Kim, M.; Gampa, G.; Carlson, B.L.; Bakken, K.K.; Mladek, A.C.; Schroeder, M.A.; et al. In Vivo Efficacy of Tesevatinib in EGFR-Amplified Patient-Derived Xenograft Glioblastoma Models May Be Limited by Tissue Binding and Compensatory Signaling. Mol. Cancer Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Mahadevan, D.; Ellsworth, R.; Cooke, L.; Bearss, D.; Stea, B. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiat. Oncol. 2009, 4, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loilome, W.; Joshi, A.D.; ap Rhys, C.M.; Piccirillo, S.; Vescovi, A.L.; Gallia, G.L.; Riggins, G.J. Glioblastoma cell growth is suppressed by disruption of Fibroblast Growth Factor pathway signaling. J. Neurooncol. 2009, 94, 359–366. [Google Scholar] [CrossRef]
- Liffers, K.; Kolbe, K.; Westphal, M.; Lamszus, K.; Schulte, A. Histone Deacetylase Inhibitors Resensitize EGFR/EGFRvIII-Overexpressing, Erlotinib-Resistant Glioblastoma Cells to Tyrosine Kinase Inhibition. Target. Oncol. 2016, 11, 29–40. [Google Scholar] [CrossRef]
- Schlaff, C.D.; Arscott, W.T.; Gordon, I.; Tandle, A.; Tofilon, P.; Camphausen, K. Radiosensitization Effects of Novel Triple-Inhibitor CUDC-101 in Glioblastoma Multiforme and Breast Cancer Cells In Vitro. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, S650. [Google Scholar] [CrossRef]
- Sharma, P.; Sonawane, P.; Herpai, D.; D’Agostino, R.; Rossmeisl, J.; Tatter, S.; Debinski, W. Multireceptor targeting of glioblastoma. Neurooncol. Adv. 2020, 2, vdaa107. [Google Scholar] [CrossRef]
- Drappatz, J.; Norden, A.D.; Wong, E.T.; Doherty, L.M.; Lafrankie, D.C.; Ciampa, A.; Kesari, S.; Sceppa, C.; Gerard, M.; Phan, P.; et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 85–90. [Google Scholar] [CrossRef]
- Chheda, M.G.; Wen, P.Y.; Hochberg, F.H.; Chi, A.S.; Drappatz, J.; Eichler, A.F.; Yang, D.; Beroukhim, R.; Norden, A.D.; Gerstner, E.R.; et al. Vandetanib plus sirolimus in adults with recurrent glioblastoma: Results of a phase I and dose expansion cohort study. J. Neurooncol. 2015, 121, 627–634. [Google Scholar] [CrossRef]
- Lee, E.Q.; Kaley, T.J.; Duda, D.G.; Schiff, D.; Lassman, A.B.; Wong, E.T.; Mikkelsen, T.; Purow, B.W.; Muzikansky, A.; Ancukiewicz, M.; et al. A Multicenter, Phase II, Randomized, Noncomparative Clinical Trial of Radiation and Temozolomide with or without Vandetanib in Newly Diagnosed Glioblastoma Patients. Clin. Cancer Res. 2015, 21, 3610–3618. [Google Scholar] [CrossRef] [Green Version]
- Schiff, D.; Desjardins, A.; Cloughesy, T.; Mikkelsen, T.; Glantz, M.; Chamberlain, M.C.; Reardon, D.A.; Wen, P.Y. Phase 1 dose escalation trial of the safety and pharmacokinetics of cabozantinib concurrent with temozolomide and radiotherapy or temozolomide after radiotherapy in newly diagnosed patients with high-grade gliomas. Cancer 2016, 122, 582–587. [Google Scholar] [CrossRef]
- Raymond, E.; Brandes, A.A.; Dittrich, C.; Fumoleau, P.; Coudert, B.; Clement, P.M.; Frenay, M.; Rampling, R.; Stupp, R.; Kros, J.M.; et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J. Clin. Oncol. 2008, 26, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Zustovich, F.; Landi, L.; Lombardi, G.; Porta, C.; Galli, L.; Fontana, A.; Amoroso, D.; Galli, C.; Andreuccetti, M.; Falcone, A.; et al. Sorafenib plus daily low-dose temozolomide for relapsed glioblastoma: A phase II study. Anticancer Res. 2013, 33, 3487–3494. [Google Scholar] [CrossRef]
- Den, R.B.; Kamrava, M.; Sheng, Z.; Werner-Wasik, M.; Dougherty, E.; Marinucchi, M.; Lawrence, Y.R.; Hegarty, S.; Hyslop, T.; Andrews, D.W.; et al. A phase I study of the combination of sorafenib with temozolomide and radiation therapy for the treatment of primary and recurrent high-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, N.; Gielen, G.H.; Kebir, S.; Wieland, A.; Till, A.; Mack, F.; Schaub, C.; Tzaridis, T.; Reinartz, R.; Niessen, M.; et al. Phase I trial of dovitinib (TKI258) in recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2016, 142, 1581–1589. [Google Scholar] [CrossRef]
- Sharma, M.; Schilero, C.; Peereboom, D.M.; Hobbs, B.P.; Elson, P.; Stevens, G.H.J.; McCrae, K.; Nixon, A.B.; Ahluwalia, M.S. Phase II study of Dovitinib in recurrent glioblastoma. J. Neurooncol. 2019, 144, 359–368. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Lamborn, K.R.; Robins, H.I.; Mehta, M.P.; Chang, S.M.; Butowski, N.A.; Deangelis, L.M.; Abrey, L.E.; Zhang, W.T.; Prados, M.D.; et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010, 12, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Alshareef, M.; Porto, G.B.F.; Infinger, L.K.; Vandergrift, W.A., 3rd; Lindhorst, S.M.; Varma, A.K.; Patel, S.J.; Cachia, D. Preconditioning with INC280 and LDK378 drugs sensitizes MGMT-unmethylated glioblastoma to temozolomide: Pre-clinical assessment. J. Neurol. Sci. 2020, 418, 117102. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Dong, M.; Cheng, F.; Mao, F.; Zong, W.; Wu, K.; Wang, H.; Xie, R.; Wang, B.; Lei, T.; et al. LRIG2 promotes the proliferation and cell cycle progression of glioblastoma cells in vitro and in vivo through enhancing PDGFRβ signaling. Int. J. Oncol. 2019, 54, 2257. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Gigas, D.C.; Kesari, S.; Drappatz, J.; Kim, R.; Zimmerman, J.; Ostrowsky, L.; Wen, P.Y. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology 2006, 67, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Ilagan, E.; Manning, B.D. Emerging role of mTOR in the response to cancer therapeutics. Trends Cancer 2016, 2, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Aksoy, O.; Wong, R.A.; Ilkhanizadeh, S.; Novotny, C.J.; Gustafson, W.C.; Truong, A.Y.; Cayanan, G.; Simonds, E.F.; Haas-Kogan, D.; et al. A Kinase Inhibitor Targeted to mTORC1 Drives Regression in Glioblastoma. Cancer Cell 2017, 31, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Pillai, R.N.; Ramalingam, S.S. Inhibition of insulin-like growth factor receptor: End of a targeted therapy? Transl. Lung Cancer Res. 2013, 2, 14–22. [Google Scholar] [CrossRef]
- Beckwith, H.; Yee, D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol. Endocrinol. 2015, 29, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, J.P.; Eisenmann, C.; Klumpp, A.; Weller, M. Co-inhibition of epidermal growth factor receptor and type 1 insulin-like growth factor receptor synergistically sensitizes human malignant glioma cells to CD95L-induced apoptosis. Biochem. Biophys. Res. Commun. 2004, 321, 524–530. [Google Scholar] [CrossRef]
- Viswanathan, A.; Musa, A.; Murugesan, A.; Vale, J.R.; Afonso, C.A.M.; Konda Mani, S.; Yli-Harja, O.; Candeias, N.R.; Kandhavelu, M. Battling Glioblastoma: A Novel Tyrosine Kinase Inhibitor with Multi-Dimensional Anti-Tumor Effect (Running Title: Cancer Cells Death Signalling Activation). Cells 2019, 8, 1624. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, J.J.; Fu, X.L.; Guang, R.; To, S.T. Advances in the targeting of HIF-1α and future therapeutic strategies for glioblastoma multiforme (Review). Oncol. Rep. 2017, 37, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Wilky, B.A.; Rudek, M.A.; Ahmed, S.; Laheru, D.A.; Cosgrove, D.; Donehower, R.C.; Nelkin, B.; Ball, D.; Doyle, L.A.; Chen, H.; et al. A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours. Br. J. Cancer 2015, 112, 24–31. [Google Scholar] [CrossRef] [Green Version]
- El Meskini, R.; Iacovelli, A.J.; Kulaga, A.; Gumprecht, M.; Martin, P.L.; Baran, M.; Householder, D.B.; Van Dyke, T.; Weaver Ohler, Z. A preclinical orthotopic model for glioblastoma recapitulates key features of human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. Dis. Model. Mech. 2015, 8, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Sunayama, J.; Matsuda, K.; Sato, A.; Tachibana, K.; Suzuki, K.; Narita, Y.; Shibui, S.; Sakurada, K.; Kayama, T.; Tomiyama, A.; et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells 2010, 28, 1930–1939. [Google Scholar] [CrossRef]
- Schreck, K.C.; Allen, A.N.; Wang, J.; Pratilas, C.A. Combination MEK and mTOR inhibitor therapy is active in models of glioblastoma. Neurooncol. Adv. 2020, 2, vdaa138. [Google Scholar] [CrossRef]
- Gravina, G.L.; Mancini, A.; Colapietro, A.; Delle Monache, S.; Sferra, R.; Pompili, S.; Vitale, F.; Martellucci, S.; Marampon, F.; Mattei, V.; et al. The Brain Penetrating and Dual TORC1/TORC2 Inhibitor, RES529, Elicits Anti-Glioma Activity and Enhances the Therapeutic Effects of Anti-Angiogenetic Compounds in Preclinical Murine Models. Cancers 2019, 11, 1604. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Debinski, W. Receptor-Targeted Glial Brain Tumor Therapies. Int. J. Mol. Sci. 2018, 19, 3326. [Google Scholar] [CrossRef] [Green Version]
- Rossmeisl, J.H.; Herpai, D.; Quigley, M.; Cecere, T.E.; Robertson, J.L.; D’Agostino, R.B.; Hinckley, J.; Tatter, S.B.; Dickinson, P.J.; Debinski, W. Phase I trial of convection-enhanced delivery of IL13RA2 and EPHA2 receptor targeted cytotoxins in dogs with spontaneous intracranial gliomas. Neuro Oncol. 2021, 23, 422–434. [Google Scholar] [CrossRef]
- Gundogdu, E.; Demir, E.S.; Özgenç, E.; Yeğen, G.; Aksu, B. Applying Quality by Design Principles in the Development and Preparation of a New Radiopharmaceutical: Technetium-99m-Imatinib Mesylate. ACS Omega 2020, 5, 5297–5305. [Google Scholar] [CrossRef]
- Glekas, A.P.; Pillarsetty, N.K.; Punzalan, B.; Khan, N.; Smith-Jones, P.; Larson, S.M. In vivo imaging of Bcr-Abl overexpressing tumors with a radiolabeled imatinib analog as an imaging surrogate for imatinib. J. Nucl. Med. 2011, 52, 1301–1307. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, C.; Ogawa, M.; Kumata, K.; Fujinaga, M.; Kato, K.; Yamasaki, T.; Yui, J.; Kawamura, K.; Hatori, A.; Fukumura, T.; et al. [11C]sorafenib: Radiosynthesis and preliminary PET study of brain uptake in P-gp/Bcrp knockout mice. Bioorg. Med. Chem. Lett. 2011, 21, 2220–2223. [Google Scholar] [CrossRef]
- Saleem, A.; Searle, G.E.; Kenny, L.M.; Huiban, M.; Kozlowski, K.; Waldman, A.D.; Woodley, L.; Palmieri, C.; Lowdell, C.; Kaneko, T.; et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Basuli, F.; Wu, H.; Li, C.; Shi, Z.-D.; Sulima, A.; Griffiths, G.L. A first synthesis of 18F-radiolabeled lapatinib: A potential tracer for positron emission tomographic imaging of ErbB1/ErbB2 tyrosine kinase activity. J. Label. Compd. Radiopharm. 2011, 54, 633–636. [Google Scholar] [CrossRef]
- Lien, V.T.; Celen, S.; Nuruddin, S.; Attili, B.; Doumont, G.; Van Simaeys, G.; Bormans, G.; Klaveness, J.; Olberg, D.E. Preclinical evaluation of [(18)F]cabozantinib as a PET imaging agent in a prostate cancer mouse model. Nucl. Med. Biol. 2020, 93, 74–80. [Google Scholar] [CrossRef]
- Gao, M.; Lola, C.M.; Wang, M.; Miller, K.D.; Sledge, G.W.; Zheng, Q.H. Radiosynthesis of [11C]Vandetanib and [11C]chloro-Vandetanib as new potential PET agents for imaging of VEGFR in cancer. Bioorg. Med. Chem. Lett. 2011, 21, 3222–3226. [Google Scholar] [CrossRef]
- Li, F.; Jiang, S.; Zu, Y.; Lee, D.Y.; Li, Z. A tyrosine kinase inhibitor-based high-affinity PET radiopharmaceutical targets vascular endothelial growth factor receptor. J. Nucl. Med. 2014, 55, 1525–1531. [Google Scholar] [CrossRef] [Green Version]
- Lagas, J.S.; van Waterschoot, R.A.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther. 2010, 9, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef] [Green Version]
- Batsios, G.; Viswanath, P.; Subramani, E.; Najac, C.; Gillespie, A.M.; Santos, R.D.; Molloy, A.R.; Pieper, R.O.; Ronen, S.M. PI3K/mTOR inhibition of IDH1 mutant glioma leads to reduced 2HG production that is associated with increased survival. Sci. Rep. 2019, 9, 10521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs, D.H.; Sarker, D. Pharmacodynamic Biomarker Development for PI3K Pathway Therapeutics. Transl. Oncogenom. 2015, 7, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, S.M.; Ray, P. Non-invasive imaging of PI3K/Akt/mTOR signalling in cancer. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 418–431. [Google Scholar] [PubMed]
- Makino, A.; Arai, T.; Hirata, M.; Ono, M.; Ohmomo, Y.; Saji, H. Development of novel PET probes targeting phosphatidylinositol 3-kinase (PI3K) in tumors. Nucl. Med. Biol. 2016, 43, 101–107. [Google Scholar] [CrossRef]
- Ferris, T.; Carroll, L.; Jenner, S.; Aboagye, E.O. Use of radioiodine in nuclear medicine-A brief overview. J. Label. Compd. Radiopharm. 2021, 64, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Guagnano, V.; Furet, P.; Spanka, C.; Bordas, V.; Le Douget, M.; Stamm, C.; Brueggen, J.; Jensen, M.R.; Schnell, C.; Schmid, H.; et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J. Med. Chem. 2011, 54, 7066–7083. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients with FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef]
- Wu, Y.M.; Su, F.; Kalyana-Sundaram, S.; Khazanov, N.; Ateeq, B.; Cao, X.; Lonigro, R.J.; Vats, P.; Wang, R.; Lin, S.F.; et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013, 3, 636–647. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.H.; Wecksler, A.T.; Zhang, G.; Morisseau, C.; Nguyen, L.V.; Fu, S.H.; Hammock, B.D. Synthesis and biological evaluation of sorafenib- and regorafenib-like sEH inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3732–3737. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [Green Version]
- Gerisch, M.; Hafner, F.T.; Lang, D.; Radtke, M.; Diefenbach, K.; Cleton, A.; Lettieri, J. Mass balance, metabolic disposition, and pharmacokinetics of a single oral dose of regorafenib in healthy human subjects. Cancer Chemother. Pharmacol. 2018, 81, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Ettrich, T.J.; Seufferlein, T. Regorafenib. Recent Results Cancer Res. 2018, 211, 45–56. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.K.; Relias, V.; Sebastian, S.; Jayaraman, V.; Saif, M.W. Management of regorafenib-related toxicities: A review. Ther. Adv. Gastroenterol. 2015, 8, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Simard, J.R.; Getlik, M.; Grütter, C.; Pawar, V.; Wulfert, S.; Rabiller, M.; Rauh, D. Development of a fluorescent-tagged kinase assay system for the detection and characterization of allosteric kinase inhibitors. J. Am. Chem Soc. 2009, 131, 13286–13296. [Google Scholar] [CrossRef]
- Kort, A.; Durmus, S.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Brain and Testis Accumulation of Regorafenib is Restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm. Res. 2015, 32, 2205–2216. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Yamada, T.; Arai, S.; Fukuda, K.; Taniguchi, H.; Tanimoto, A.; Nishiyama, A.; Takeuchi, S.; Yamashita, K.; Ohtsubo, K.; et al. Distribution and Activity of Lenvatinib in Brain Tumor Models of Human Anaplastic Thyroid Cancer Cells in Severe Combined Immune Deficient Mice. Mol. Cancer Ther. 2019, 18, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e302–e310. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Hoelder, S.; Clarke, P.A.; Workman, P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol. Oncol. 2012, 6, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Tsou, H.R.; Overbeek-Klumpers, E.G.; Hallett, W.A.; Reich, M.F.; Floyd, M.B.; Johnson, B.D.; Michalak, R.S.; Nilakantan, R.; Discafani, C.; Golas, J.; et al. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J. Med. Chem. 2005, 48, 1107–1131. [Google Scholar] [CrossRef]
- Nasrazadani, A.; Brufsky, A. Neratinib: The emergence of a new player in the management of HER2+ breast cancer brain metastasis. Future Oncol. 2020, 16, 247–254. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9915743, Neratinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Neratinib (accessed on 26 May 2021).
- Zhao, X.Q.; Xie, J.D.; Chen, X.G.; Sim, H.M.; Zhang, X.; Liang, Y.J.; Singh, S.; Talele, T.T.; Sun, Y.; Ambudkar, S.V.; et al. Neratinib reverses ATP-binding cassette B1-mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol. Pharmacol. 2012, 82, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Prové, A.; Dirix, L. Neratinib for the treatment of breast cancer. Expert Opin. Pharmacother. 2016, 17, 2243–2248. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Królicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with (213)Bi-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; Herndon, J.E., 2nd; McLendon, R.E.; Pegram, C.N.; Provenzale, J.M.; Quinn, J.A.; et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: Phase II study results. J. Clin. Oncol. 2006, 24, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. 225Ac- and 213Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Schibli, R. Prospects in folate receptor-targeted radionuclide therapy. Front. Oncol. 2013, 3, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Radionuclide | Half-Life | Energy (keV) | Function |
|---|---|---|---|
| 11C | 20.4 min | 960 (β+) | PET |
| 13N | 9.96 min | 1190 (β+) | PET |
| 15O | 2.07 min | 1720 (β+) | PET |
| 18F | 119 min | 640 (β+) | PET |
| 123I | 13.2 h | 159 (γ) | SPECT |
| 125I | 60.1 h | 15 (Auger) | Therapy |
| 131I | 8 d | 365 (γ), 606 (β−) | SPECT and Therapy |
| Radionuclide | Half-Life | Energy (keV) | Function |
|---|---|---|---|
| Diagnostic | |||
| 64Cu | 12.7 h | 656 (β+) | PET |
| 67Ga | 78.3 h | 6.26 (Auger); | SPECT (Therapy) |
| 93, 184, 300, 393 (γ) | |||
| 68Ga | 67.7 min | 1899 (β+) | PET |
| 86Y | 14.7 h | 1221 (β+) | PET |
| 89Zr | 78.4 h | 902 (β+) | PET |
| 99mTc | 6.02 h | 140 (γ) | SPECT |
| 111In | 67.2 h | 6.75 (Auger); 171, 245 (γ) | SPECT (Therapy) |
| 44Sc | 3.97 | 632 (β+) | PET |
| Therapeutic | |||
| 67Cu | 2.58 d | 141 (β−) | β-Therapy |
| 91, 93, 185 (γ) | |||
| 89Sr | 52.7 d | 1463 (β−) | β-Therapy |
| 90Y | 64 h | 2280 (β−) | β-Therapy |
| 117mSn | 13.6 d | 150 (β−) | β-Therapy |
| 153Sm | 46.5 h | 640; 710; 808 (β−) | β-Therapy |
| 103 (γ) | |||
| 161Tb | 6.89 d | 154 (β−) | β/AE-Therapy |
| 49, 75 (γ) | |||
| ≤50 (AE) | |||
| 166Ho | 26.8 h | 665 (β−) | β-Therapy |
| 81 (γ) | |||
| 169Er | 9.4 d | 350 (β−) | β-Therapy |
| 177Lu | 6.75 d | 176, 384, 497 (β−) | β-Therapy |
| 113; 208 (γ) | |||
| 186Re | 3.7 d | 1069 (β−) | β-Therapy |
| 137 (γ) | |||
| 188Re | 17 h | 2120 (β−) | β-Therapy |
| 155 (γ) | |||
| 211At | 7.2 h | 5870 (α) | α-Therapy |
| 212Pb | 10.2 h | 570 (β−); | α-Therapy |
| 6050, 6090 (α—from 212Bi daughter) | |||
| 238, 300 (γ) | |||
| 213Bi | 45.6 min | 5558, 5875 (α) | α-Therapy |
| 324 (γ) | |||
| 223Ra | 11.4 d | 5433 (α) | α-Therapy |
| 144, 154, 269, 324, 338 (γ) | |||
| 225Ac | 10 d | 5830, 5792, 5790, 5732 (α) | α-Therapy |
| 86, 440 (γ) | |||
| 47Sc | 3.35 d | 162 (β−) | β-Therapy |
| Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference | |
|---|---|---|---|---|
| Olaratumab (IMC-3G3) | mAb | II (completed-no results) (ramucirumab) | NCT00895180 [67] | |
| II (+/−) (bevacizumab) | [228] | |||
| Tandutinib (MLN518) | SM | I/II (−) | [296] | |
| Nilotinib (AMN107) | SM | II (completed, no results) | NCT01140568 [67,297] | |
| Imatinib (Gleevec) | SM |  | See Table 9 | |
| Dasatinib (BMS-354825) | SM | |||
| Regorafenib | SM | |||
| Sorafenib | SM | |||
| Sunitinib | SM | |||
| Ponatinib | SM | |||
| Nintedanib (BIBF 1120) | SM | |||
| Lenvatinib (E7080) | SM | |||
| Dovitinib (TKI258) | SM | |||
| Pazopanib (GW786034) | SM | |||
| Compound | Type | Clinical Trials: Phase, Overall Conclusion (+) or (−), (Combined Therapy) | Reference | |
|---|---|---|---|---|
| Erdafitinib (JNJ-42756493) | SM | I (+) | [321] | |
| I (+) (advanced or refractory solid tumors) | [325] | |||
| Futibatinib (TAS-120) | SM | I (+) (advanced solid tumors) | [323] | |
| I/II (active, not recruiting) | NCT02052778 [67] | |||
| Infigratinib (BGJ398) | SM | II (completed, no results) | NCT01975701 [67] | |
| AZD4547 | SM | I/II (completed, no results) | NCT02824133 [67] | |
| Ponatinib (AP24534) | SM |  | See Table 9 | |
| Dovitinib (TKI258) | SM | |||
| Nintedanib (BIBF 1120) | SM | |||
| Lenvatinib (E7080) | SM | |||
| Anlotinib (AL3818) | SM | |||
| Regorafenib (BAY73-4506) | SM | |||
| Inclusion Criteria |
OR
|
| Exclusion Criteria |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolcaen, J.; Nair, S.; Driver, C.H.S.; Boshomane, T.M.G.; Ebenhan, T.; Vandevoorde, C. Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma. Pharmaceuticals 2021, 14, 626. https://doi.org/10.3390/ph14070626
Bolcaen J, Nair S, Driver CHS, Boshomane TMG, Ebenhan T, Vandevoorde C. Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma. Pharmaceuticals. 2021; 14(7):626. https://doi.org/10.3390/ph14070626
Chicago/Turabian StyleBolcaen, Julie, Shankari Nair, Cathryn H. S. Driver, Tebatso M. G. Boshomane, Thomas Ebenhan, and Charlot Vandevoorde. 2021. "Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma" Pharmaceuticals 14, no. 7: 626. https://doi.org/10.3390/ph14070626
APA StyleBolcaen, J., Nair, S., Driver, C. H. S., Boshomane, T. M. G., Ebenhan, T., & Vandevoorde, C. (2021). Novel Receptor Tyrosine Kinase Pathway Inhibitors for Targeted Radionuclide Therapy of Glioblastoma. Pharmaceuticals, 14(7), 626. https://doi.org/10.3390/ph14070626