2-Styrylchromones: Cytotoxicity and Modulation of Human Neutrophils’ Oxidative Burst
Abstract
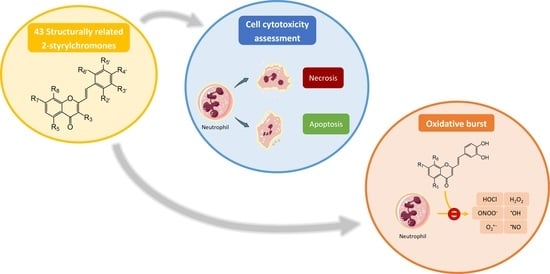
:1. Introduction
2. Results
2.1. Effect of 2-SC on Neutrophils’ Viability
2.2. Effect of 2-SC on Neutrophils’ Oxidative Burst
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Equipment
4.3. Methods
4.3.1. General Information
4.3.2. Human Neutrophils Isolation
4.3.3. Assessment of the Effect of 2-SC on Neutrophils’ Viability
4.3.4. Assessment of the Effect of 2-SC on Neutrophils’ Oxidative Burst
4.3.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Elbim, C.; Lizard, G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytom. A 2009, 75A, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. [Google Scholar]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Freitas, M.; Lima, J.L.F.C.; Fernandes, E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review. Anal. Chim. Acta 2009, 649, 8–23. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- El Kebir, D.; Filep, J.G. Modulation of neutrophil apoptosis and the resolution of inflammation through β2 Iintegrins. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many ways to die. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Gabai, V.L. Cell death and survival assays. In Chaperones: Methods and Protocols; Methods in Molecular Biology; Calderwood, S.K., Prince, T.L., Eds.; Humana Press: New York, NY, USA, 2018; pp. 107–127. [Google Scholar]
- Santos, C.M.M.; Silva, A.M.S. An overview of 2-styrylchromones: Natural occurrence, synthesis, reactivity and biological properties. Eur. J. Org. Chem. 2017, 2017, 3115–3133. [Google Scholar] [CrossRef]
- Doria, G.; Romeo, C.; Forgione, A.; Sberze, P.; Tibolla, N.; Corno, M.L.; Cruzzola, G.; Cadelli, G. Antiallergic agents. III. Substituted trans-2-ethenyl-4-oxo-4H-1-benzopyran-6-carboxylic acids. Eur. J. Med. Chem. 1979, 14, 347–351. [Google Scholar] [CrossRef]
- Desideri, N.; Conti, C.; Mastromarino, P.; Mastropaolo, F. Synthesis and anti-rhinovirus activity of 2-styrylchromones. Antivir. Chem. Chemother. 2000, 11, 373–381. [Google Scholar] [CrossRef]
- Desideri, N.; Mastromarino, P.; Conti, C. Synthesis and evaluation of antirhinovirus activity of 3-hydroxy and 3-methoxy 2-styrylchromones. Antivir. Chem. Chemother. 2003, 14, 195–203. [Google Scholar] [CrossRef]
- Conti, C.; Mastromarino, P.; Goldoni, P.; Portalone, G.; Desideri, N. Synthesis and anti-rhinovirus properties of fluoro-substituted flavonoids. Antivir. Chem. Chemother. 2005, 16, 267–276. [Google Scholar] [CrossRef]
- Ujwala, B.; Priyadarsini, P.; Rao, V.M. Synthesis and bio-activity evaluation of 2-styrylchromones. Int. J. Pharma Bio Sci. 2013, 4, 199–206. [Google Scholar]
- Nikam, M.D.; Mahajan, P.S.; Damale, M.G.; Sangshetti, J.N.; Chate, A.V.; Dabhade, S.K.; Gill, C.H. Novel amalgamation of 2-styrylchromones and 1,2,4-triazole: Synthesis, antimicrobial evaluation and docking study. Lett. Drug. Des. Discov. 2015, 12, 650–660. [Google Scholar] [CrossRef]
- Rao, V.M.; Ujwala, B.; Priyadarsini, P.; Krishna Murthy, P. Synthesis, antioxidant and antimicrobial activity of three new 2-styrylchromones and their analogues. Der Pharma Chem. 2016, 8, 1–6. [Google Scholar]
- Momin, M.; Ramjugernath, D.; Chenia, H.; Koorbanally, N.A. Synthesis and evaluation of novel fluorinated 2-styrylchromones as antibacterial agents. J. Chem. 2013, 2013, 436758. [Google Scholar] [CrossRef] [Green Version]
- Takao, K.; Endo, S.; Nagai, J.; Kamauchi, H.; Takemura, Y.; Uesawa, Y.; Sugita, Y. 2-Styrylchromone derivatives as potent and selective monoamine oxidase B inhibitors. Bioorganic Chem. 2019, 92, 103285. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiao, L.; Xie, D.; Yuan, Y.; Chen, N.; Dai, J.; Guo, S. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Jung, H.A.; Min, B.S.; Choi, J.S. Anticholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory compounds from the heartwood of Juniperus chinensis. Chem. Pharm. Bull. 2015, 63, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Lima, J.L.F.C. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorganic Med. Chem. 2007, 15, 6027–6036. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Capela, J.P.; Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Pinto, D.C.G.A.; Santos, C.M.M.; Cavaleiro, J.A.S.; Lima, J.L.F.C.; Fernandes, E. Inhibition of NF-kB activation and cytokines production in THP-1 monocytes by 2-styrylchromones. Med. Chem. 2015, 11, 560–566. [Google Scholar] [CrossRef]
- Takamatsu, S.; Nagle, D.G.; Gerwick, W.H. Secondary metabolites from marine Cyanobacteria and Algae inhibit LFA-1/ICAM-1 mediated cell adhesion. Planta Med. 2004, 70, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lu, P.-J.; Yang, C.-N.; Hulme, C.; Shaw, A.Y. Structure-activity relationship study of growth inhibitory 2-styrylchromones against carcinoma cells. Med. Chem. Res. 2013, 22, 2385–2394. [Google Scholar] [CrossRef]
- Momoi, K.; Sugita, Y.; Ishihara, M.; Satoh, K.; Kikuchi, H.; Hashimoto, K.; Yokoe, I.; Nishikawa, H.; Seiichiro, F.; Sakagami, H. Cytotoxic activity of styrylchromones against human tumor cell lines. In Vivo 2005, 19, 157–163. [Google Scholar]
- Shaw, A.Y.; Chang, C.-Y.; Liau, H.-H.; Lu, P.-J.; Chen, H.-L.; Yang, C.-N.; Li, H.-Y. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur. J. Med. Chem. 2009, 44, 2552–2562. [Google Scholar] [CrossRef]
- Lee, K.Y.; Nam, D.H.; Moon, C.S.; Seo, S.H.; Lee, J.Y.; Lee, Y.S. Synthesis and anticancer activity of lavendustin A derivatives containing arylethenylchromone substituents. Eur. J. Med. Chem. 2006, 41, 991–996. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Porto, G.; Fernandes, E. Modulation of human neutrophils’ oxidative burst by flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Ribeiro, D.; Tomé, S.M.; Silva, A.M.S.; Fernandes, E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur. J. Med. Chem. 2014, 86, 153–164. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.-C.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Yang, C.-H.; Yang, Y.; Liu, J.-H. Platachromone A–D: Cytotoxic 2-styrylchromones from the bark of Platanus×acerifolia (Aiton) Willd. Phytochem. Lett. 2013, 6, 387–391. [Google Scholar] [CrossRef]
- Uesawa, Y.; Nagai, J.; Shi, H.; Sakagami, H.; Bandow, K.; Tomomura, A.; Tomomura, M.; Endo, S.; Takao, K.; Sugita, Y. Quantitative structure–cytotoxicity relationship of 2-styrylchromones. Anticancer Res. 2019, 39, 6489. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.P.R.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Garcia, M.B.Q.; Silva, A.M.S.; Pinto, D.C.G.A.; Santos, C.M.M.; Cavaleiro, J.A.S.; Lima, J.L.F.C. Cyclic voltammetric analysis of 2-styrylchromones: Relationship with the antioxidant activity. Bioorg. Med. Chem. 2008, 16, 7939–7943. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S.; Cavaleiro, J.A.S. Synthesis of new hydroxy-2-styrylchromones. Eur. J. Org. Chem. 2003, 2003, 4575–4585. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S.; Cavaleiro, J.A.S. Efficient syntheses of new polyhydroxylated 2,3-diaryl-9H-xanthen-9-ones. Eur. J. Org. Chem. 2009, 2009, 2642–2660. [Google Scholar] [CrossRef]
- Freitas, M.; Costa, V.M.; Ribeiro, D.; Couto, D.; Porto, G.; Carvalho, F.; Fernandes, E. Acetaminophen prevents oxidative burst and delays apoptosis in human neutrophils. Toxicol. Lett. 2013, 219, 170–177. [Google Scholar] [CrossRef] [PubMed]







| 2-SC | Concentration (μM) | 2-SC | Concentration (μM) |
|---|---|---|---|
| A1 | 75.00 | C2 | 25.00 |
| A2 | 6.25 | C3 | 50.00 |
| A3 | 75.00 | C4 | 37.50 |
| A4 | 25.00 | C5 | 75.00 |
| A5 | 6.25 | C6 | 37.50 |
| A6 | 100.00 | C7 | 25.00 |
| A7 | 6.25 | C8 | 6.25 |
| A8 | 25.00 | D1 | 12.50 |
| B1 | 25.00 | D2 | 12.50 |
| B2 | 6.25 | D3 | 12.50 |
| B3 | 25.00 | E1 | 25.00 |
| B4 | 6.25 | E2 | 12.50 |
| B5 | 6.25 | F1 | 6.25 |
| B6 | 12.50 | F2 | 12.50 |
| B7 | 0.38 | G1 | 12.50 |
| B8 | 25.00 | G2 | 12.50 |
| B9 | 12.50 | G3 | 25.00 |
| B10 | 6.25 | G4 | 25.00 |
| B11 | 12.50 | H1 | 12.50 |
| B12 | 12.50 | H2 | 50.00 |
| B13 | 12.50 | H3 | 12.50 |
| C1 | 25.00 |
| 2-SC | R5 | R7 | R8 | R2′ | R3′ | R4′ | R6′ | Inhibitory Activity * (% ± SEM) or IC50 (μM, mean ± SEM) | |
|---|---|---|---|---|---|---|---|---|---|
| A1 |  | - | - | - | - | - | - | - | <30% 75.00µM |
| A2 | OH | - | - | - | - | - | - | <30% 6.25µM | |
| A3 | - | OH | - | - | - | - | - | 53 ± 4 % 75.00µM | |
| A4 | OH | OH | - | - | - | - | - | <30% 25.00µM | |
| A5 | OCH3 | - | - | - | - | - | - | <30% 6.25µM | |
| A6 | - | - | - | - | OH | - | - | <30% 100.00µM | |
| A7 | OH | - | - | - | OH | - | - | <30% 6.25µM | |
| A8 | OH | - | - | OH | - | - | - | <30% 25.00µM | |
| B1 |  | - | - | - | - | - | - | - | 7.5 ± 0.4 |
| B2 | OH | - | - | - | - | - | - | 50 ± 3 % 6.25µM | |
| B3 | - | OH | - | - | - | - | - | 4.1 ± 0.3 | |
| B4 | OH | OH | - | - | - | - | - | 3.1 ± 0.3 | |
| B5 | OH | OCH3 | - | - | - | - | - | <30% 6.25µM | |
| B6 | - | - | - | - | OH | - | - | 1.0 ± 0.1 | |
| B7 | OH | - | - | - | OH | - | - | <30% 0.38µM | |
| B8 | - | OH | - | - | OH | - | - | 0.7 ± 0.1 | |
| B9 | OH | OH | - | - | OH | - | - | 0.8 ± 0.1 | |
| B10 | - | OCH3 | - | - | OH | - | - | 1.0 ± 0.1 | |
| B11 | OCH3 | OCH3 | - | - | OH | - | - | 0.8 ± 0.1 | |
| B12 | - | OH | OH | - | OH | - | - | 0.9 ± 0.3 | |
| B13 | - | OCH3 | OCH3 | - | OH | - | - | 1.0 ± 0.2 | |
| C1 |  | - | - | - | - | - | - | - | <30% 25.00µM |
| C2 | - | - | - | - | OCH3 | - | - | <30% 25.00µM | |
| C3 | OCH3 | - | - | - | OCH3 | - | - | 54 ± 4 % 50.00µM | |
| C4 | - | OCH3 | - | - | OCH3 | - | - | 24.2 ± 0.3 | |
| C5 | OCH3 | OCH3 | - | - | OCH3 | - | - | 54 ± 2 % 50.00µM | |
| C6 | - | OCH3 | OCH3 | - | OCH3 | - | - | 20 ± 2 | |
| C7 | - | OBn | OBn | - | OCH3 | - | - | <30% 25.00µM | |
| C8 | - | OH | OH | - | OCH3 | - | - | 1.4 ± 0.2 | |
| D1 |  | - | - | - | - | OBn | - | - | <30% 12.50µM |
| D2 | OCH3 | OCH3 | - | - | OBn | - | - | <30% 12.50µM | |
| D3 | - | OCH3 | OCH3 | - | OBn | - | - | <30% 12.50µM | |
| E1 |  | - | - | - | - | - | - | - | <30% 6.25µM |
| E2 | OH | - | - | - | - | - | - | <30% 12.50µM | |
| F1 |  | - | - | - | - | - | - | - | <30% 6.25µM |
| F2 | OH | - | - | - | - | - | - | <30% 12.50µM | |
| G1 |  | OH | - | - | - | Cl | - | - | <30% 12.50µM |
| G2 | - | - | - | - | - | Cl | - | <30% 12.50µM | |
| G3 | OH | - | - | - | Cl | Cl | - | <30% 25.00µM | |
| G4 | OH | - | - | Cl | - | - | Cl | <30% 25.00µM | |
| H1 |  | - | - | - | - | - | - | - | <30% 12.50µM |
| H2 | OCH3 | - | - | - | - | - | - | <30% 50.00µM | |
| H3 | - | - | - | - | OCH3 | OCH3 | - | <30% 12.50µM | |
 | Positive control Quercetin | 0.8 ± 0.1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, M.; Freitas, M.; Zanchetta, M.; Silva, A.M.S.; Fernandes, E.; Ribeiro, D. 2-Styrylchromones: Cytotoxicity and Modulation of Human Neutrophils’ Oxidative Burst. Pharmaceuticals 2022, 15, 288. https://doi.org/10.3390/ph15030288
Lucas M, Freitas M, Zanchetta M, Silva AMS, Fernandes E, Ribeiro D. 2-Styrylchromones: Cytotoxicity and Modulation of Human Neutrophils’ Oxidative Burst. Pharmaceuticals. 2022; 15(3):288. https://doi.org/10.3390/ph15030288
Chicago/Turabian StyleLucas, Mariana, Marisa Freitas, Marco Zanchetta, Artur M. S. Silva, Eduarda Fernandes, and Daniela Ribeiro. 2022. "2-Styrylchromones: Cytotoxicity and Modulation of Human Neutrophils’ Oxidative Burst" Pharmaceuticals 15, no. 3: 288. https://doi.org/10.3390/ph15030288
APA StyleLucas, M., Freitas, M., Zanchetta, M., Silva, A. M. S., Fernandes, E., & Ribeiro, D. (2022). 2-Styrylchromones: Cytotoxicity and Modulation of Human Neutrophils’ Oxidative Burst. Pharmaceuticals, 15(3), 288. https://doi.org/10.3390/ph15030288