Effects of an Extract of the Brown Seaweed Ascophylum nodosum on Postprandial Glycaemic Control in Healthy Subjects: A Randomized Controlled Study
Abstract
:1. Introduction
2. Results
2.1. Participant Characteristics
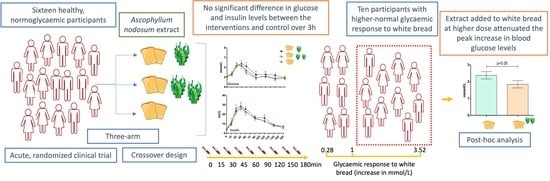
2.2. Effects on Postprandial Glucose Levels
2.3. Effects on Postprandial Insulin Levels
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participants
4.3. Study Protocol
4.4. Anthropometric, Body Composition, Blood Pressure, and Heart Rate Measurements
4.5. Blood Collection and Biochemical Analyses
4.6. Saliva Collection and Analysis
4.7. Intervention and Control Meal
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed]
- Tufford, A.R.; Calder, P.C.; Van’t Veer, P.; Feskens, E.F.; Ockhuizen, T.; Kraneveld, A.D.; Sikkema, J.; de Vries, J. Is nutrition science ready for the twenty-first century? Moving towards transdisciplinary impacts in a changing world. Eur. J. Nutr. 2020, 59 (Suppl. 1), 1–10. [Google Scholar] [CrossRef] [PubMed]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021; Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- FAO. Food Balance Sheets—A Handbook; Food and Agricultural Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Sun, Q. Intake of whole grain foods and risk of type 2 diabetes: Results from three prospective cohort studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Swaminathan, S.; Dehghan, M.; Raj, J.M.; Thomas, T.; Rangarajan, S.; Jenkins, D.; Mony, P.; Mohan, V.; Lear, S.A.; Avezum, A.; et al. Associations of cereal grains intake with cardiovascular disease and mortality across 21 countries in Prospective Urban and Rural Epidemiology study: Prospective cohort study. BMJ 2021, 372, m4948. [Google Scholar] [CrossRef]
- Toups, K.E. Global approaches to promoting whole grain consumption. Nutr. Rev. 2020, 78 (Suppl. 1), 54–60. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Diaz, R.; Rosengren, A.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.A.; Augustin, L.S.A.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M.S.; et al. Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: A Systematic Review and Updated Meta-Analyses of Prospective Cohort Studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Henry, C.J. Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 670–702. [Google Scholar] [CrossRef] [PubMed]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.C.; Karim, M.R.; Yusoff, M.B.; Tan, S.H.; Asras, M.F.; Rashid, S.S. New insights into seaweed polyphenols on glucose homeostasis. Pharm. Biol. 2015, 53, 1087–1097. [Google Scholar] [CrossRef]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [PubMed]
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goni, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of edible brown algae extracts for the inhibition of intestinal carbohydrate digestive enzymes involved in glucose release from the diet. J. Nutr. Sci. 2021, 10, e5. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae-a Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, Y.J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Kim, H.K. Ecklonia cava Inhibits Glucose Absorption and Stimulates Insulin Secretion in Streptozotocin-Induced Diabetic Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 439294. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Bonham, M.P.; Ryan, L. Do marine algal polyphenols have antidiabetic, antihyperlipidemic or anti-inflammatory effects in humans? A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2039–2054. [Google Scholar] [CrossRef]
- Paradis, M.E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Dalla Man, C.; Campioni, M.; Basu, A.; Klee, G.; Toffolo, G.; Cobelli, C.; Rizza, R.A. Effects of age and sex on postprandial glucose metabolism: Differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 2006, 55, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur. J. Clin. Nutr. 2012, 66, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Simper, T.N.; Morris, C.; Lynn, A.; O’Hagan, C.; Kilner, K. Responses to oral glucose challenge differ by physical activity volume and intensity: A pilot study. J. Sport Health Sci. 2020, 9, 645–650. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Hancock, D.; Petocz, P.; Brand-Miller, J.C. The physiologic and phenotypic significance of variation in human amylase gene copy number. Am. J. Clin. Nutr. 2018, 108, 737–748. [Google Scholar] [CrossRef]
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bell, D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, K.; Futami-Suda, S.; Yano, H.; Motoyama, M.; Matsumura, N.; Igari, Y.; Suzuki, T.; Nakano, H.; Oba, K. The effects of postprandial glucose and insulin levels on postprandial endothelial function in subjects with normal glucose tolerance. Cardiovasc. Diabetol. 2012, 11, 98. [Google Scholar] [CrossRef]
- Mamo, J.C.; Yu, K.C.; Elsegood, C.L.; Smith, D.; Vine, D.; Gennat, H.C.; Voevodin, M.; Proctor, S.D. Is atherosclerosis exclusively a postprandial phenomenon? Clin. Exp. Pharmacol. Physiol. 1997, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Bjorck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.; Perelman, D.; Breschi, A.; Limcaoco, P.; Kellogg, R.; McLaughlin, T.; Snyder, M. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018, 16, e2005143. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, E.; Kang, I.; Lee, M.; Lee, Y. Anti-diabetic effects and anti-inflammatory effects of Laminaria japonica and Hizikia fusiforme in skeletal muscle: In vitro and in vivo model. Nutrients 2018, 10, 491. [Google Scholar] [CrossRef]
- Zhang, J.; Tiller, C.; Shen, J.; Wang, C.; Girouard, G.S.; Dennis, D.; Barrow, C.J.; Miao, M.; Ewart, H.S. Antidiabetic properties of polysaccharide- and polyphenolic-enriched fractions from the brown seaweed Ascophyllum nodosum. Can. J. Physiol. Pharmacol. 2007, 85, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.Y.; Shin, Y.E.; Kim, H.K. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res. 2019, 65, 54–62. [Google Scholar] [CrossRef]
- Zaharudin, N.; Tullin, M.; Pekmez, C.T.; Sloth, J.J.; Rasmussen, R.R.; Dragsted, L.O. Effects of brown seaweeds on postprandial glucose, insulin and appetite in humans—A randomized, 3-way, blinded, cross-over meal study. Clin. Nutr. 2021, 40, 830–838. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Choi, H.; Jun, H.S. The Effect of Phloroglucinol, A Component of Ecklonia cava Extract, on Hepatic Glucose Production. Mar. Drugs 2017, 15, 106. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Extraction, purification, chemical characterization and antioxidant properties in vitro of polyphenols from the brown macroalga Ascophyllum nodosum. Algal Res. 2023, 70, 102989. [Google Scholar] [CrossRef]
- Wascher, T.C.; Schmoelzer, I.; Wiegratz, A.; Stuehlinger, M.; Mueller-Wieland, D.; Kotzka, J.; Enderle, M. Reduction of postchallenge hyperglycaemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur. J. Clin. Investig. 2005, 35, 551–557. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012, 10, 2604. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. A Single-Dose of a Polyphenol-Rich Fucus Vesiculosus Extract is Insufficient to Blunt the Elevated Postprandial Blood Glucose Responses Exhibited by Healthy Adults in the Evening: A Randomised Crossover Trial. Antioxidants 2019, 8, 49. [Google Scholar] [CrossRef]
- Palmu, S.; Rehunen, S.; Kautiainen, H.; Eriksson, J.G.; Korhonen, P.E. Body surface area and glucose tolerance—The smaller the person, the greater the 2-hour plasma glucose. Diabetes Res. Clin. Pract. 2019, 157, 107877. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Daimiel, L.; Ordo vas, J.M. Personalized nutrition and cardiovascular disease prevention: From Framingham to PREDIMED. Adv. Nutr. 2014, 5, 368S–371S. [Google Scholar] [CrossRef] [PubMed]
- van Ommen, B.; Keijer, J.; Kleemann, R.; Elliott, R.; Drevon, C.A.; McArdle, H.; Gibney, M.; Muller, M. The challenges for molecular nutrition research 2: Quantification of the nutritional phenotype. Genes Nutr. 2008, 3, 51–59. [Google Scholar] [CrossRef]
- Gibney, E.R. Personalised nutrition—Phenotypic and genetic variation in response to dietary intervention. Proc. Nutr. Soc. 2020, 79, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.; Lara, J.; Mathers, J.C. Personalising nutritional guidance for more effective behaviour change. Proc. Nutr. Soc. 2015, 74, 130–138. [Google Scholar] [CrossRef]
- Curtis, P.J.; Adamson, A.J.; Mathers, J.C. Effects on nutrient intake of a family-based intervention to promote increased consumption of low-fat starchy foods through education, cooking skills and personalised goal setting: The Family Food and Health Project. Br. J. Nutr. 2012, 107, 1833–1844. [Google Scholar] [CrossRef]
- Yang, Z.M.; Lin, J.; Chen, L.H.; Zhang, M.; Chen, W.W.; Yang, X.R. The roles of AMY1 copies and protein expression in human salivary alpha-amylase activity. Physiol. Behav. 2015, 138, 173–178. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, e000327. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nakamichi, N.; Sekino, H.; Nakano, S. Comparison of the effects of acarbose and voglibose in healthy subjects. Clin. Ther. 1997, 19, 720–729. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Dominguez Diaz, L.; Fernandez-Ruiz, V.; Camara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Miletic, I.D.; Schiffman, S.S.; Miletic, V.D.; Sattely-Miller, E.A. Salivary IgA secretion rate in young and elderly persons. Physiol. Behav. 1996, 60, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. Biomed. Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Panel on Dietetic Products Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]




| Characteristics | Mean (SD) | Range |
|---|---|---|
| N | 16 | na |
| Male/Female | 7/9 | na |
| Age (years) | 29.6 (±10.4) | 18–57 |
| Body weight (kg) | 73.85 (±11.4) | 60.5–93.4 |
| BMI (kg/m2) | 24.5 (±3.9) | 18.1–32.1 |
| DBP (mmHg) | 110.4 (±9.2) | 90–125 |
| SDP (mmHg) | 69.2 (±8.2) | 52–82 |
| Heart rate | 68.6 (±11.5) | 49–95 |
| TG (mmol/L) | 0.88 (±0.53) | 0.39–2.26 |
| TCHOL (mmol/L) | 4.67 (±0.91) | 3.47–6.31 |
| HDL (mmol/L) | 1.21 (±0.22) | 0.79–1.49 |
| LDL (mmol/L) | 2.44 (±0.69) | 1.29–3.49 |
| Fasting plasma glucose (mmol/L) | 5.11 (±0.32) | 4.31–5.6 |
| Salivary flow (mL/min) | 0.393 (±0.22) | 0.154–0.867 |
| Salivary α-amylase activity (U/mL) | 66.5 (±46.94) | 16.63–181.53 |
| Salivary α-amylase secretion rate (U/min) | 37.73 (±30.03) | 2.63–136.66 |
| Parameters | “Lower-Normal” | “Higher-Normal” | Difference |
|---|---|---|---|
| Cmax | 5.64 (±0.38) | 7.47 (±0.75) | p < 0.0001 |
| iCmax | 0.57 (±0.29) | 2.38 (±0.69) | p < 0.0001 |
| iAUC180 | 19.83 (±25.72) | 115.3 (±57.85) | p = 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konic Ristic, A.; Ryan, S.; Attjioui, M.; O’Connell, S.; Gibney, E.R. Effects of an Extract of the Brown Seaweed Ascophylum nodosum on Postprandial Glycaemic Control in Healthy Subjects: A Randomized Controlled Study. Mar. Drugs 2023, 21, 337. https://doi.org/10.3390/md21060337
Konic Ristic A, Ryan S, Attjioui M, O’Connell S, Gibney ER. Effects of an Extract of the Brown Seaweed Ascophylum nodosum on Postprandial Glycaemic Control in Healthy Subjects: A Randomized Controlled Study. Marine Drugs. 2023; 21(6):337. https://doi.org/10.3390/md21060337
Chicago/Turabian StyleKonic Ristic, Aleksandra, Sinead Ryan, Maha Attjioui, Shane O’Connell, and Eileen R. Gibney. 2023. "Effects of an Extract of the Brown Seaweed Ascophylum nodosum on Postprandial Glycaemic Control in Healthy Subjects: A Randomized Controlled Study" Marine Drugs 21, no. 6: 337. https://doi.org/10.3390/md21060337
APA StyleKonic Ristic, A., Ryan, S., Attjioui, M., O’Connell, S., & Gibney, E. R. (2023). Effects of an Extract of the Brown Seaweed Ascophylum nodosum on Postprandial Glycaemic Control in Healthy Subjects: A Randomized Controlled Study. Marine Drugs, 21(6), 337. https://doi.org/10.3390/md21060337