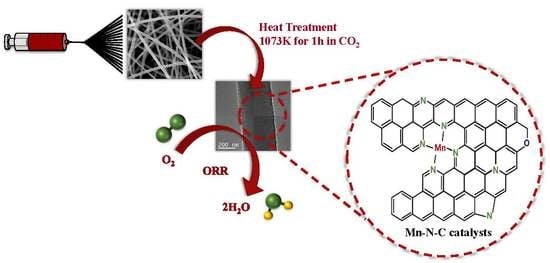
Fabrication of Mn-N-C Catalyst for Oxygen Reduction Reactions Using Mn-Embedded Carbon Nanofiber
Abstract
:1. Introduction
2. Materials and Methods
3. Results
Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, B.H.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 7, 1700363. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Kim, C.; Choi, H.-J.; Gwon, O.; Jung, S.-M.; Seo, J.-M.; Cho, S.-J.; Ju, Y.-W.; Jeong, H.Y.; et al. Fe@C2N: A highly-efficient indirect-contact oxygen reduction catalyst. Nano Energy 2018, 44, 304–310. [Google Scholar] [CrossRef]
- Ng, W.; Yang, Y.; Van Der Veen, K.; Rothenberg, G.; Yan, N. Enhancing the performance of 3D porous N-doped carbon in oxygen reduction reaction and supercapacitor via boosting the meso-macropore interconnectivity using the “exsolved” dual-template. Carbon 2018, 129, 293–300. [Google Scholar] [CrossRef]
- Guo, J.; Gadipelli, S.; Yang, Y.; Li, Z.; Lu, Y.; Brett, D.J.L.; Guo, Z.X. An efficient carbon-based ORR catalyst from low-temperature etching of ZIF-67 with ultra-small cobalt nanoparticles and high yield. J. Mater. Chem. A 2019, 7, 3544–3551. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.-W.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-C.; Zhao, S.; Tang, D.; Hou, P.-X.; Liu, C.; Cheng, H.-M.; Zhang, F. A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions. Energy Environ. Sci. 2016, 9, 3079–3084. [Google Scholar] [CrossRef]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High Performance Fe- and N-Doped Carbon Catalyst with Graphene Structure for Oxygen Reduction. Sci. Rep. 2013, 3, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, K.; Hashimoto, K.; Nakanishi, S. Instantaneous one-pot synthesis of Fe–N-modified graphene as an efficient electrocatalyst for the oxygen reduction reaction in acidic solutions. Chem. Commun. 2012, 48, 10213. [Google Scholar] [CrossRef]
- Ju, Y.; Yoo, S.; Kim, C.; Kim, S.; Jeon, I.; Shin, J.; Baek, J.-B.; Kim, G. Fe@N-Graphene Nanoplatelet-Embedded Carbon Nanofibers as Efficient Electrocatalysts for Oxygen Reduction Reaction. Adv. Sci. 2015, 3, 1500205. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, D.; Liu, S.; Chen, S.; Hanif, M.; Hou, H. Free-standing nitrogen-doped carbon nanotubes at electrospun carbon nanofibers composite as an efficient electrocatalyst for oxygen reduction. Electrochim. Acta 2014, 138, 318–324. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.; Niu, L.; Zhou, X.-G.; Lu, T.-H.; Yuan, W.-K. Ultrasonic synthesis of nitrogen-doped carbon nanofibers as platinum catalyst support for oxygen reduction. J. Power Sources 2011, 196, 9356–9360. [Google Scholar] [CrossRef]
- Ren, G.; Lu, X.; Li, Y.; Zhu, Y.; Dai, L.; Jiang, L. Porous Core–Shell Fe3C Embedded N-doped Carbon Nanofibers as an Effective Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 4118–4125. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Sun, Z.; You, T. Free-standing nitrogen-doped carbon nanofiber films as highly efficient electrocatalysts for oxygen reduction. Nanoscale 2013, 5, 9528. [Google Scholar] [CrossRef]
- Fan, L.; Xu, Y.; Zhou, X.; Chen, F.; Fu, Q. Effect of salt concentration in spinning solution on fiber diameter and mechanical property of electrospun styrene-butadiene-styrene tri-block copolymer membrane. Polym. 2018, 153, 61–69. [Google Scholar] [CrossRef]
- Zhang, C.; Yue, X.; Wu, L.; Han, Y.; Sheng, J. Study on morphology of electrospun poly(vinylalcohol) mats. Eur. Polym. J. 2005, 41, 423–432. [Google Scholar]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Kim, K.-S.; Park, S.-J. Synthesis and high electrochemical capacitance of N-doped microporous carbon/carbon nanotubes for supercapacitor. J. Electroanal. Chem. 2012, 673, 58–64. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2012, 23, 2436–2444. [Google Scholar] [CrossRef]
- Cao, W. Preparation of Highly-Active Oxygen Reduction Reaction Catalyst by Direct Co-Pyrolysis of Biomass with KOH. Int. J. Electrochem. Sci. 2019, 14, 250–261. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc-air batteries’ cathodes. Nano Energy 2017, 40, 382–389. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Fan, L.; Han, J.; Xiong, Y. Fe/Ni-N-CNFs electrochemical catalyst for oxygen reduction reaction/oxygen evolution reaction in alkaline media. Appl. Surf. Sci. 2017, 401, 89–99. [Google Scholar] [CrossRef]
- Wang, Z.; Zuo, P.; Fan, L.; Han, J.; Xiong, Y.; Yin, G. Facile electrospinning preparation of phosphorus and nitrogen dual-doped cobalt-based carbon nanofibers as bifunctional electrocatalyst. J. Power Sources 2016, 311, 68–80. [Google Scholar] [CrossRef]
- Peng, H.; Liu, F.; Liu, X.; Liao, S.; You, C.; Tian, X.; Nan, H.; Luo, F.; Song, H.; Fu, Z.; et al. Effect of Transition Metals on the Structure and Performance of the Doped Carbon Catalysts Derived From Polyaniline and Melamine for ORR Application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, C.; Dai, Y.; Xie, J. Pore structure control of mesoporous carbon as supercapacitor material. Mater. Lett. 2007, 61, 4639–4642. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Zhang, C. A Three-Dimensional Carbon Nanotube/Graphene Sandwich and Its Application as Electrode in Supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Dubal, D.; Gund, G.S.; Lokhande, C.D.; Holze, R. Controlled Growth of CoSxNanostrip Arrays (CoSx-NSA) on Nickel Foam for Asymmetric Supercapacitors. Energy Technol. 2014, 2, 401–408. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Ju, Y.-W. Fabrication of Mn-N-C Catalyst for Oxygen Reduction Reactions Using Mn-Embedded Carbon Nanofiber. Energies 2020, 13, 2561. https://doi.org/10.3390/en13102561
Kim H-Y, Ju Y-W. Fabrication of Mn-N-C Catalyst for Oxygen Reduction Reactions Using Mn-Embedded Carbon Nanofiber. Energies. 2020; 13(10):2561. https://doi.org/10.3390/en13102561
Chicago/Turabian StyleKim, Hyo-Young, and Young-Wan Ju. 2020. "Fabrication of Mn-N-C Catalyst for Oxygen Reduction Reactions Using Mn-Embedded Carbon Nanofiber" Energies 13, no. 10: 2561. https://doi.org/10.3390/en13102561
APA StyleKim, H. -Y., & Ju, Y. -W. (2020). Fabrication of Mn-N-C Catalyst for Oxygen Reduction Reactions Using Mn-Embedded Carbon Nanofiber. Energies, 13(10), 2561. https://doi.org/10.3390/en13102561