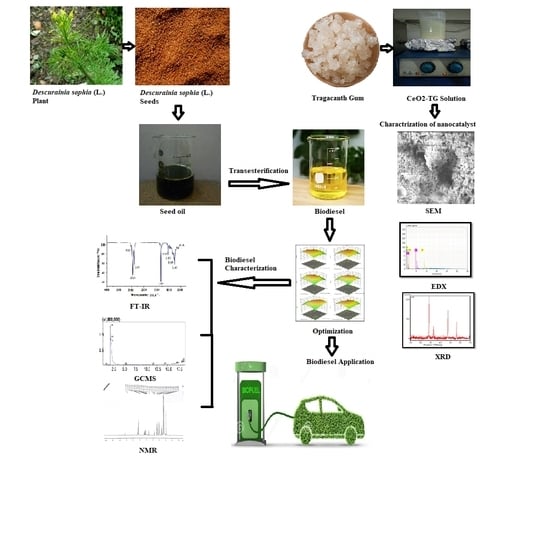
Sustainable Production of Biodiesel from Novel Non-Edible Oil Seeds (Descurainia sophia L.) via Green Nano CeO2 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Study of Feedstock
2.2. Nanocatalyst Synthesis
2.3. Characterization of Synthesized Nanocatalyst
2.4. Biodiesel Production and Analysis
2.5. Experimental Design for Optimization Process
2.6. Physicochemical Properties Evaluation of Biodiesel
2.7. Catalyst Reusability Evaluation
3. Results and Discussion
3.1. D. sophia Seed Oil and FFA Content
3.2. Characterization of TG-CeO2 Nanocatalyst
3.2.1. XRD Analysis
3.2.2. Morphological Evaluation and Elemental Analysis
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Thermogravimetric Analysis (TGA)
3.2.5. Functional Groups Analysis via FT-IR
3.3. Model Validation and Optimization of DSBD via RSM-CCD
3.4. Reaction Parameters Consequence on Biodiesel Yield
3.4.1. Combined Effect of Catalyst Concentration and Reaction Temperature
3.4.2. Combined Effect of Catalyst Concentration and the Molar Ratio of Oil to Methanol
3.4.3. Combined Effect of Catalyst Concentration and Reaction Time
3.4.4. Combined Effect of Reaction Temperature and the Molar Ratio of Oil to Methanol
3.4.5. Combined Effect of Reaction Temperature and Reaction Time
3.4.6. Combined Effect of Molar Ratio of Oil to Methanol and Reaction Time
3.5. D. sophia (L.) Biodiesel Characterization
3.5.1. FT-IR Spectroscopy Study of Biodiesel
3.5.2. GCMS Analysis of Biodiesel
3.5.3. NMR Analysis of Biodiesel
3.6. Fuel Properties of D. sophia Biodiesel in Comparison with International Standards
3.7. Catalyst Reusability
3.8. Limitations and Future Aspects of the Present Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chozhavendhan, S.; Singh, M.V.P.; Fransila, B.; Kumar, R.P.; Devi, G.K. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1, 1–6. [Google Scholar] [CrossRef]
- Suresh, T.; Sivarajasekar, N.; Balasubramani, K. Enhanced ultrasonic assisted biodiesel production from meat industry waste (pig tallow) using green copper oxide nanocatalyst: Comparison of response surface and neural network modelling. Renew. Energy 2021, 164, 897–907. [Google Scholar] [CrossRef]
- Noshadi, I.; Amin, N.; Parnas, R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Khan, I.W.; Naeem, A.; Farooq, M.; Ghazi, Z.A.; Saeed, T.; Perveen, F.; Malik, T. Biodiesel production by valorizing waste non-edible wild olive oil using heterogeneous base catalyst: Process optimization and cost estimation. Fuel 2022, 320, 123828. [Google Scholar] [CrossRef]
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Nabi, M.N. The potential of utilising papaya seed oil and stone fruit kernel oil as non-edible feedstock for biodiesel production in Australia—A review. Energy Rep. 2019, 5, 280–297. [Google Scholar] [CrossRef]
- Cheema, S.I.; Ahmad, M.; Ullah, R.; Mothana, R.A.; Noman, O.M.; Zafar, M.; Sultana, S.; Hameed, A.; Naz, S.; Akhtar, M.T. Implication, visualization, and characterization through scanning electron microscopy as a tool to identify nonedible oil seeds. Microsc. Res. Tech. 2021, 84, 379–393. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Performance of functionalized magnetic nanocatalysts and feedstocks on biodiesel production: A review study. J. Clean. Prod. 2021, 305, 127200. [Google Scholar] [CrossRef]
- Vicente, G.; Martinez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour. Technol. 2007, 98, 1724–1733. [Google Scholar] [CrossRef]
- HadiNezhad, M.; Rowland, O.; Hosseinian, F. The fatty acid profile and phenolic composition of Descurainia sophia seeds extracted by supercritical CO2. J. Am. Oil Chem. Soc. 2015, 92, 1379–1390. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.; Khalid, M.; Abdullah, E.; Nolasco-Hipolito, C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019, 7, 103219. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid–base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Ahmad, M.; Asma, M.; Munir, M.; Zafar, M.; Sultana, S.; Mujtaba, M.; Mohamed, A.; Kalam, M.A. Efficient Production of Wild and Non-Edible Brassica juncea (L.) Czern. Seed Oil into High-Quality Biodiesel via Novel, Green and Recyclable NiSO4 Nano-Catalyst. Sustainability 2022, 14, 10188. [Google Scholar] [CrossRef]
- Hosseini, S.A. Nanocatalysts for biodiesel production. Arab. J. Chem. 2022, 15, 104152. [Google Scholar] [CrossRef]
- Bano, S.; Ganie, A.S.; Sultana, S.; Sabir, S.; Khan, M.Z. Fabrication and optimization of nanocatalyst for biodiesel production: An overview. Front. Energy Res. 2020, 8, 579014. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Hydary, I.A.; Al-Hattab, T.A. Nano-magnetic catalyst CaO-Fe3O4 for biodiesel production from date palm seed oil. Bull. Chem. React. Eng. Catal. 2017, 12, 460–468. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, X.; Yang, J. Synthesis of CaO-CeO2 catalysts by soft template method for biodiesel production. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 23–24 October 2017; p. 012048. [Google Scholar]
- Ebadinezhad, B.; Haghighi, M.; Zeinalzadeh, H. Carbon-templated meso-design of nanostructured CeAPSO-34 for biodiesel production from free fatty acid and waste oil. Renew. Energy 2022, 195, 716–733. [Google Scholar] [CrossRef]
- Zandi-Atashbar, N.; Ensafi, A.A.; Ahoor, A.H. Nano-CeO2/SiO2 as an efficient catalytic conversion of waste engine oil into liquid fuel. J. Clean. Prod. 2017, 166, 1010–1019. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Nazarnezhad, S.; Mousavi, S.H.; Sadegh Amiri, M.; Darroudi, M.; Baino, F.; Kargozar, S. Gum tragacanth (GT): A versatile biocompatible material beyond borders. Molecules 2021, 26, 1510. [Google Scholar] [CrossRef]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum Tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.T.; Ahmad, M.; Shaheen, A.; Zafar, M.; Ullah, R.; Asma, M.; Sultana, S.; Munir, M.; Rashid, N.; Malik, K. Comparative study of liquid biodiesel from Sterculia foetida (bottle tree) using CuO-CeO2 and Fe2O3 nano catalysts. Front. Energy Res. 2019, 7, 4. [Google Scholar] [CrossRef]
- Ferrero, G.O.; Faba, E.M.S.; Rickert, A.A.; Eimer, G.A. Alternatives to rethink tomorrow: Biodiesel production from residual and non-edible oils using biocatalyst technology. Renew. Energy 2020, 150, 128–135. [Google Scholar] [CrossRef]
- Velusamy, P.; Das, J.; Pachaiappan, R.; Vaseeharan, B.; Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind. Crops Prod. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Souza, M.C.G.; de Oliveira, M.F.; Vieira, A.T.; de Faria, A.M.; Batista, A.C.F. Methylic and ethylic biodiesel production from crambe oil (Crambe abyssinica): New aspects for yield and oxidative stability. Renew. Energy 2021, 163, 368–374. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Navada, M.K.; Karnikkar, N.G.; D’Souza, J.N.; Kouser, S.; Aroor, G.; Kudva, J.; Jayappa, M.D. Biosynthesis of phyto functionalized cerium oxide nanoparticles mediated from Scoparia dulsis L. for appraisal of anti-cancer potential against adenocarcinomic lung cancer cells and paracetamol sensing potentiality. Environ. Sci. Pollut. Res. 2022, 1–20. [Google Scholar] [CrossRef]
- Ahmad, A.; Javed, M.S.; Khan, S.; Almutairi, T.M.; Mohammed, A.A.; Luque, R. Green synthesized Ag decorated CeO2 nanoparticles: Efficient photocatalysts and potential antibacterial agents. Chemosphere 2023, 310, 136841. [Google Scholar] [CrossRef]
- Irfan, M.; Munir, H.; Ismail, H. Moringa oleifera gum based silver and zinc oxide nanoparticles: Green synthesis, characterization and their antibacterial potential against MRSA. Biomater. Res. 2021, 25, 1–8. [Google Scholar] [CrossRef]
- Anjum, F.; Gul, S.; Khan, M.I.; Khan, M.A. Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes. Green Process. Synth. 2020, 9, 63–76. [Google Scholar] [CrossRef]
- Khaledi, K.; Haghighi, M.; Sadeghpour, P. On the catalytic properties and performance of core-shell ZSM-5@ MnO nanocatalyst used in conversion of methanol to light olefins. Microporous Mesoporous Mater. 2017, 246, 51–61. [Google Scholar] [CrossRef]
- Akintelu, S.; Folorunso, A. Characterization and antimicrobial investigation of synthesized silver nanoparticles from Annona muricata leaf extracts. J. Nanotechnol. Nanomed. Nanobiotechnol. 2019, 6, 1–5. [Google Scholar]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Ala’a, H.; Osman, A.I.; Kumar, P.S.M.; Jamil, F.; Al-Haj, L.; Al Nabhani, A.; Kyaw, H.H.; Myint, M.T.Z.; Mehta, N.; Rooney, D.W. Circular economy approach of enhanced bifunctional catalytic system of CaO/CeO2 for biodiesel production from waste loquat seed oil with life cycle assessment study. Energy Convers. Manag. 2021, 236, 114040. [Google Scholar]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Application of walnut shell ash/ZnO/K2CO3 as a new composite catalyst for biodiesel generation from Moringa oleifera oil. Fuel 2022, 311, 122624. [Google Scholar] [CrossRef]
- Thangarasu, V.; Siddharth, R.; Ramanathan, A. Modeling of process intensification of biodiesel production from Aegle Marmelos Correa seed oil using microreactor assisted with ultrasonic mixing. Ultrason. Sonochem. 2020, 60, 104764. [Google Scholar] [CrossRef]
- Abu-Ghazala, A.H.; Abdelhady, H.H.; Mazhar, A.A.; El-Deab, M.S. Valorization of hazard waste: Efficient utilization of white brick waste powder in the catalytic production of biodiesel from waste cooking oil via RSM optimization process. Renew. Energy 2022, 200, 1120–1133. [Google Scholar] [CrossRef]
- Pang, D.; Tan, H.; Zhu, R.; Ouyang, F. Producing biodiesel from waste animal oil by modified ZnO. Int. J. Green Energy 2017, 14, 703–711. [Google Scholar] [CrossRef]
- Attari, A.; Abbaszadeh-Mayvan, A.; Taghizadeh-Alisaraie, A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 2022, 158, 106357. [Google Scholar] [CrossRef]
- Helmi, M.; Tahvildari, K.; Hemmati, A.; Azar, P.A.; Safekordi, A. Converting waste cooking oil into biodiesel using phosphomolybdic acid/clinoptilolite as an innovative green catalyst via electrolysis procedure; optimization by response surface methodology (RSM). Fuel Process. Technol. 2022, 225, 107062. [Google Scholar] [CrossRef]
- Abd Malek, M.N.F.; Pushparaja, L.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploration of efficiency of nano calcium oxide (CaO) as catalyst for enhancement of biodiesel production. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3935. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]
- Rehan, M.; Gardy, J.; Demirbas, A.; Rashid, U.; Budzianowski, W.; Pant, D.; Nizami, A. Waste to biodiesel: A preliminary assessment for Saudi Arabia. Bioresour. Technol. 2018, 250, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, I.M.; Paes, O.A.; Maia, P.J.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S., Jr.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Sandhya, R.; Velavan, R.; Ravichandran, J. Biodiesel production from waste cooking oil using copper doped zinc oxide nanocatalyst–process optimisation and economic analysis. Int. J. Oil Gas Coal Technol. 2020, 25, 488–497. [Google Scholar] [CrossRef]
- Elkelawy, M.; Bastawissi, H.A.-E.; Esmaeil, K.K.; Radwan, A.M.; Panchal, H.; Sadasivuni, K.K.; Ponnamma, D.; Walvekar, R. Experimental studies on the biodiesel production parameters optimization of sunflower and soybean oil mixture and DI engine combustion, performance, and emission analysis fueled with diesel/biodiesel blends. Fuel 2019, 255, 115791. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A. Optimization of biodiesel production from mixture of edible and nonedible vegetable oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Munawaroh, H.S.H.; Lam, M.K.; Khoo, K.S. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef]
- Chaudhry, B.; Akhtar, M.S.; Ahmad, M.; Munir, M.; Zafar, M.; Alhajeri, N.S.; Ala’a, H.; Ahmad, Z.; Hasan, M.; Bokhari, A. Membrane based reactors for sustainable treatment of Coronopus didymus L. by developing Iodine doped potassium oxide membrane under Dynamic conditions. Chemosphere 2022, 303, 135138. [Google Scholar] [CrossRef]
- Chakraborty, M.; Baruah, D. Production and characterization of biodiesel obtained from Sapindus mukorossi kernel oil. Energy 2013, 60, 159–167. [Google Scholar] [CrossRef]
- Atabani, A.; Shobana, S.; Mohammed, M.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Ala’a, H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Hazmi, B.; Ibrahim, M.L.; Tsubota, T.; Alharthi, F.A. Potential heterogeneous nano-catalyst via integrating hydrothermal carbonization for biodiesel production using waste cooking oil. Chemosphere 2022, 286, 131913. [Google Scholar] [CrossRef]
- Yahya, S.I.; Aghel, B. Estimation of kinematic viscosity of biodiesel-diesel blends: Comparison among accuracy of intelligent and empirical paradigms. Renew. Energy 2021, 177, 318–326. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Munir, M.; Saeed, M.; Ahmad, M.; Waseem, A.; Alsaady, M.; Asif, S.; Ahmed, A.; Khan, M.S.; Bokhari, A.; Mubashir, M. Cleaner production of biodiesel from novel non-edible seed oil (Carthamus lanatus L.) via highly reactive and recyclable green nano CoWO3@ rGO composite in context of green energy adaptation. Fuel 2023, 332, 126265. [Google Scholar] [CrossRef]
- Zhukova, Y.; Studenyak, Y.; Mariychuk, R. New Indicators for Determination of Acid Number in Diesel Fuel Containing Biodiesel. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 431–443. [Google Scholar]
- Khan, I.U.; Haleem, A.; Khan, A.U. Non-edible plant seeds of Acacia farnesiana as a new and effective source for biofuel production. RSC Adv. 2022, 12, 21223–21234. [Google Scholar] [CrossRef]
- Pali, H.S.; Sharma, A.; Kumar, N.; Singh, Y. Biodiesel yield and properties optimization from Kusum oil by RSM. Fuel 2021, 291, 120218. [Google Scholar] [CrossRef]
- Ahmad, M.; Zafar, M. Conversion of waste seed oil of Citrus aurantium into methyl ester via green and recyclable nanoparticles of zirconium oxide in the context of circular bioeconomy approach. Waste Manag. 2021, 136, 310–320. [Google Scholar]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel production using solid acid catalysts based on metal oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the density and viscosity of biodiesel and the influence of biodiesel properties on a diesel engine fuel supply system. J. Mar. Eng. Technol. 2021, 20, 299–311. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Almutairi, A.W. A close-loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhanced biofuel recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef]
- Akubude, V.; Nwaigwe, K.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Schöggl, J.-P.; Stumpf, L.; Baumgartner, R.J. The narrative of sustainability and circular economy-A longitudinal review of two decades of research. Resour. Conserv. Recycl. 2020, 163, 105073. [Google Scholar] [CrossRef]
- Mu, D.; Seager, T.P.; Rao, P.S.C.; Park, J.; Zhao, F. A resilience perspective on biofuel production. Integr. Environ. Assess. Manag. 2011, 7, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Sweetapple, C.; Fu, G.; Farmani, R.; Butler, D. Exploring wastewater system performance under future threats: Does enhancing resilience increase sustainability? Water Res. 2019, 149, 448–459. [Google Scholar] [CrossRef]
- Giannetti, B.F.; Lopez, F.J.D.; Liu, G.; Agostinho, F.; Sevegnani, F.; Almeida, C.M. A Resilient and Sustainable World: Contributions from Cleaner Production, Circular Economy, Eco-innovation, Responsible Consumption, and Cleaner Waste Systems. J. Clean. Prod. 2022, 384, 135465. [Google Scholar] [CrossRef]
- Gatto, A.; Drago, C. A taxonomy of energy resilience. Energy Policy 2020, 136, 111007. [Google Scholar] [CrossRef]












| Run | A: Catalyst Concentration | B: Reaction Temperature | C: Oil-to-Methanol Ratio | D: Reaction Time | Yield |
|---|---|---|---|---|---|
| wt% | °C | Min | % | ||
| 1 | 0.3 | 90 | 6 | 210 | 79 |
| 2 | 0.3 | 90 | 6 | 360 | 75 |
| 3 | 0.1 | 150 | 10 | 60 | 78 |
| 4 | 0.1 | 30 | 10 | 360 | 50 |
| 5 | 0.5 | 30 | 2 | 360 | 50 |
| 6 | 0.1 | 30 | 2 | 360 | 54 |
| 7 | 0.3 | 90 | 6 | 210 | 79 |
| 8 | 0.5 | 90 | 6 | 210 | 85 |
| 9 | 0.3 | 150 | 8 | 210 | 80 |
| 10 | 0.1 | 90 | 6 | 210 | 75 |
| 11 | 0.5 | 150 | 2 | 360 | 40 |
| 12 | 0.1 | 30 | 2 | 60 | 42 |
| 13 | 0.1 | 150 | 2 | 360 | 40 |
| 14 | 0.5 | 30 | 10 | 360 | 53 |
| 15 | 0.5 | 30 | 10 | 60 | 59 |
| 16 | 0.1 | 150 | 2 | 60 | 48 |
| 17 | 0.3 | 90 | 10 | 210 | 86 |
| 18 | 0.5 | 150 | 10 | 60 | 44 |
| 19 | 0.1 | 150 | 10 | 360 | 40 |
| 20 | 0.1 | 30 | 10 | 60 | 56 |
| 21 | 0.5 | 150 | 2 | 60 | 38 |
| 22 | 0.5 | 30 | 8 | 60 | 35 |
| 23 | 0.3 | 90 | 6 | 60 | 75 |
| 24 | 0.3 | 90 | 8 | 210 | 98 |
| 25 | 0.3 | 90 | 8 | 210 | 98 |
| 26 | 0.3 | 30 | 6 | 210 | 65 |
| 27 | 0.3 | 90 | 8 | 210 | 98 |
| 28 | 0.3 | 90 | 2 | 210 | 71 |
| 29 | 0.3 | 90 | 8 | 210 | 98 |
| 30 | 0.5 | 150 | 10 | 360 | 43 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 11,235.74 | 14 | 802.55 | 10.45 | <0.0001 | Significant |
| A—Catalyst concentration | 72.00 | 1 | 72.00 | 0.9379 | 0.3482 | |
| B—Reaction Temperature | 9.39 | 1 | 9.39 | 0.1223 | 0.7314 | |
| C—Oil-to-methanol Ratio | 460.06 | 1 | 460.06 | 5.99 | 0.0271 | |
| D—Reaction time | 50.00 | 1 | 50.00 | 0.6513 | 0.4322 | |
| AB | 81.00 | 1 | 81.00 | 1.06 | 0.3206 | |
| AC | 1.0000 | 1 | 1.0000 | 0.0130 | 0.9106 | |
| AD | 156.25 | 1 | 156.25 | 2.04 | 0.1742 | |
| BC | 0.2500 | 1 | 0.2500 | 0.0033 | 0.9552 | |
| BD | 225.00 | 1 | 225.00 | 2.93 | 0.1075 | |
| CD | 324.00 | 1 | 324.00 | 4.22 | 0.0578 | |
| A² | 121.91 | 1 | 121.91 | 1.59 | 0.2268 | |
| B² | 534.24 | 1 | 534.24 | 6.96 | 0.0186 | |
| C² | 181.06 | 1 | 181.06 | 2.36 | 0.1454 | |
| D² | 364.41 | 1 | 364.41 | 4.75 | 0.0457 | |
| Residual | 1151.46 | 15 | 76.76 | |||
| Lack of Fit | 670.13 | 10 | 67.01 | 0.6961 | 0.7079 | not significant |
| Pure Error | 481.33 | 5 | 96.27 | |||
| Cor Total | 12,387.20 | 29 | R2 | 0.9070 | ||
| Std. Dev | 8.76 | Adjusted R² | 0.8203 | |||
| C.V. % | 13.60 | Predicted R² | 0.6239 | |||
| Adeq Precision | 8.8543 |
| Properties | Methods | Current Study | HSD ASTM D-951 | ASTM D-6751 | EN-14214 | China GB/T 20828-2007 |
|---|---|---|---|---|---|---|
| Color | Visual | 2 | 2.0 | 2 | - | - |
| Flashpoint °C (PMCC) | ASTM D-93 | 73.5 | 60–80 | ≥93 | ≥120 | ≥130 |
| Density@ 15 °C kg/L | ASTM D-1298 | 0.800 | 0.8343 | ≤120 | ≤120 | - |
| K. Viscosity@40 °C cSt | ASTM D-445 | 4.23 | 4.223 | 1.9–6.0 | 3.4–5.0 | - |
| Pour point °C | ASTM D-97 | −7 | - | −15–16 | - | - |
| Cloud point °C | ASTM D-2500 | −12 | - | −3–12 | - | - |
| Sulfur %wt | ASTM D-4294 | 0.0001 | 0.05 | ≤0.05 | 0.020 | ≤0.05 |
| Total Acid No.mg KOH/gm | ASTM D-974 | 0.160 | 0.8 | ≤0.5 | ≤0.5 | ≤0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.T.; Ahmad, M.; Ramadan, M.F.; Makhkamov, T.; Yuldashev, A.; Mamarakhimov, O.; Munir, M.; Asma, M.; Zafar, M.; Majeed, S. Sustainable Production of Biodiesel from Novel Non-Edible Oil Seeds (Descurainia sophia L.) via Green Nano CeO2 Catalyst. Energies 2023, 16, 1534. https://doi.org/10.3390/en16031534
Akhtar MT, Ahmad M, Ramadan MF, Makhkamov T, Yuldashev A, Mamarakhimov O, Munir M, Asma M, Zafar M, Majeed S. Sustainable Production of Biodiesel from Novel Non-Edible Oil Seeds (Descurainia sophia L.) via Green Nano CeO2 Catalyst. Energies. 2023; 16(3):1534. https://doi.org/10.3390/en16031534
Chicago/Turabian StyleAkhtar, Maryam Tanveer, Mushtaq Ahmad, Mohamed Fawzy Ramadan, Trobjon Makhkamov, Akramjon Yuldashev, Oybek Mamarakhimov, Mamoona Munir, Maliha Asma, Muhammad Zafar, and Salman Majeed. 2023. "Sustainable Production of Biodiesel from Novel Non-Edible Oil Seeds (Descurainia sophia L.) via Green Nano CeO2 Catalyst" Energies 16, no. 3: 1534. https://doi.org/10.3390/en16031534
APA StyleAkhtar, M. T., Ahmad, M., Ramadan, M. F., Makhkamov, T., Yuldashev, A., Mamarakhimov, O., Munir, M., Asma, M., Zafar, M., & Majeed, S. (2023). Sustainable Production of Biodiesel from Novel Non-Edible Oil Seeds (Descurainia sophia L.) via Green Nano CeO2 Catalyst. Energies, 16(3), 1534. https://doi.org/10.3390/en16031534