Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
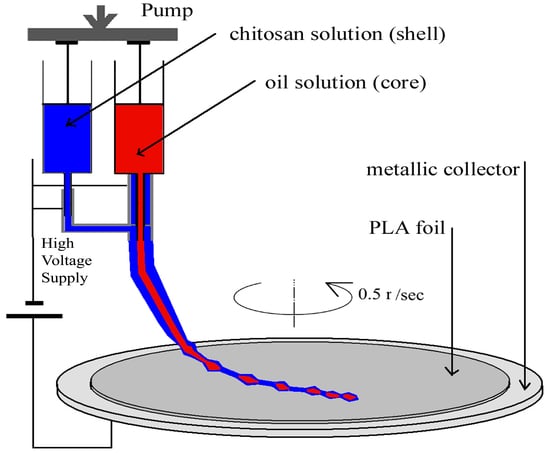
2.2. Preparation of the Nanostructured Coatings
2.3. Investigation Methods
3. Results and Discussion
3.1. Transmission Electron Microscope (TEM) Results
3.2. Atomic Force Microscopy (AFM) Results
3.3. Scanning Electron Microscopy (SEM) Results
3.4. Antibacterial Tests
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Rapa, M.; Mitelut, A.C.; Tanase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Nita, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B: Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Pâslaru, E.; Zemljic, L.F.; Sdrobis, A.; Pricope, G.M.; Vasile, C. Chitosan coatings applied to polyethylene surface to obtain food-packaging materials. Cellul. Chem. Technol. 2014, 48, 565–575. [Google Scholar]
- Vasile, C.; Darie, R.N.; Sdrobis, A.; Pâslaru, E.; Pricope, G.; Baklavaridis, A.; Munteanu, S.B.; Zuburtikudis, I. Effectiveness of chitosan as antimicrobial agent in LDPE/CS composite films as minced poultry meat packaging materials. Cellul. Chem. Technol. 2014, 48, 325–336. [Google Scholar]
- Ben Arfa, A.; Preziosi-Belloy, L.; Chalier, P.; Gontard, N. Antimicrobial paper based on a soy protein isolate or modified starch coating including carvacrol and cinnamaldehyde. J. Agric. Food Chem. 2007, 55, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, K.; Dobiáš, J.; Klaudisová, K. Effect of Packaging Films releasing antimicrobial agents on stability of food products. Czech J. Food Sci. 2009, 27, S347–S349. [Google Scholar] [CrossRef]
- Hauser, C.; Wunderlich, J. Antimicrobial packaging films with a sorbic acid based coating. Procedia Food Sci. 2011, 1, 197–202. [Google Scholar] [CrossRef]
- Gamage, G.R.; Park, H.J.; Kim, K.M. Effectiveness of antimicrobial coated oriented polypropylene/polyethylene films in sprout packaging. Food Res. Int. 2009, 42, 832–839. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Aucejo, S.; Gavara, R.; Hernández-Muñoz, P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control 2013, 30, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Gallstedt, M.; Brottman, A.; Hedenqvist, M.S. Packaging-related properties of protein- and chitosan-coated paper. Packag. Technol. Sci. 2005, 18, 161–170. [Google Scholar] [CrossRef]
- Hong, S.I.; Lee, J.W.; Son, S.M. Properties of polysaccharide-coated polypropylene films as affected by biopolymer and plasticizer types. Packag. Technol. Sci. 2005, 18, 1–9. [Google Scholar] [CrossRef]
- Ho-Lee, C.; An, D.S.; Jin-Park, H.; Lee, D.S. Wide-spectrum antimicrobial packaging materials incorporating nisin and chitosan in the coating. Packag. Technol. Sci. 2003, 16, 99–106. [Google Scholar] [CrossRef]
- Tang, Z.X.; Qian, J.Q.; Shi, L.E. Preparation of chitosan nanoparticles as carrier for immobilized enzyme. Appl. Biochem. Biotechnol. 2007, 136, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Muriel-Galet, V.; Cerisuelo, J.P.; López-Carballo, G.; Lara, M.; Gavara, R.; Hernández-Muñoz, P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012, 157, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahana, F.J. Development of active packaging containing natural antioxidants. Procedia Food Sci. 2011, 1, 224–228. [Google Scholar] [CrossRef]
- Hoagland, P.D. Biodegradable Laminated Films Fabricated from Pectin and Chitosan. U.S. Patent 5,919,574, 6 July 1999. [Google Scholar]
- Stoleru-Paslaru, E.; Tsekov, Y.; Kotsilkova, R.; Ivanov, E.; Vasile, C. Mechanical behavior at nanoscale of chitosan-coated PE surface. J. Appl. Polym. Sci. 2015, 132, 42344. [Google Scholar] [CrossRef]
- Riccardi, C.; Zanini, S.; Tassetti, D. A Polymeric Film Coating Method on A Substrate by Depositing and Subsequently Polymerizing a Monomeric Composition by Plasma Treatment. U.S. Patent US20160122585A1, 5 May 2018. [Google Scholar]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tanase, E.E.; Mitelut, A.; Ailiesei, G.L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Munteanu, B.S. Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cerisuelo, J.P.; Muriel-Galet, V.; Bermúdez, J.M.; Aucejo, S.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Mathematical model to describe the release of an antimicrobial agent from an active package constituted by carvacrol in a hydrophilic EVOH coating on a PP film. J. Food Eng. 2012, 110, 26–37. [Google Scholar] [CrossRef]
- Tihminlioglu, F.; Atik, İ.D.; Özen, B. Effect of corn-zein coating on the mechanical properties of polypropylene packaging films. J. Appl. Polym. Sci. 2011, 119, 235–241. [Google Scholar] [CrossRef]
- Naraghi, M.; Arshad, S.N.; Chasiotis, I. Molecular orientation and mechanical property size effects in electrospun polyacrylonitrile nanofibers. Polymer 2011, 52, 1612–1618. [Google Scholar] [CrossRef]
- Papkov, D.; Zou, Y.; Andalib, M.N.; Goponenko, A.; Cheng, S.Z.D.; Dzenis, Y.A. Simultaneously strong and tough ultrafine continuous nanofibers. ACS Nano 2013, 7, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Cloutier, M.; Isabelle, M.; Khalil, A. Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis 2006, 184, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kouidri, M.; Saadi, A.K.; Noui, A.; Medjahed, F. The chemical composition of argan oil. Int. J. Adv. Stud. Comput. Sci. Eng. 2015, 4, 24–28. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Seul, S.D.; Lim, J.M.; Ha, S.H.; Kim, Y.H. Adhesion Enhancement of polyurethane coated leather and polyurethane foam with plasma treatment. Korean J. Chem. Eng. 2005, 22, 745–749. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Munteanu, B.S.; Zaharescu, T.; Ioanid, G.E.; Pamfil, D. Radiation mediated bioactive compounds immobilization on polymers to obtain multifunctional food packaging materials. In Proceedings of the International Conference on Applications of Radiation Science and Technology, Vienna, Austria, 24–28 April 2017. [Google Scholar]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food. Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution Technical note. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications–A review. J. Bioeng. Biomed. Sci. 2013, 3, 1000121. [Google Scholar] [CrossRef]
- Diaz, J.E.; Barrero, A.; Márquez, M.; Loscertales, I.G. Controlled Encapsulation of Hydrophobic Liquids in Hydrophilic Polymer Nanofibers by Co-electrospinning. Adv. Funct. Mater. 2006, 16, 2110–2116. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Ni, X.; Liu, G.; Cheng, W.; Han, G. Coaxial Electrospinning and characterization of core-shell structured cellulose nanocrystal reinforced PMMA/PAN composite fibers. Materials 2017, 10, 572–588. [Google Scholar]
- He, C.L.; Huang, Z.M.; Han, X.J.; Liu, L.; Zhang, H.S.; Chen, L.S. Coaxial electrospun poly(l-Lactic Acid) ultrafine fibers for sustained drug delivery. J. Macromol. Sci. Part B Phys. 2006, 45, 515–524. [Google Scholar] [CrossRef]
- Li, T.X.; Ding, X.; Sui, X.; Tian, L.L.; Zhang, Y.; Hu, J.Y.; Yang, X.D. Sustained Release of Protein Particle Encapsulated in Bead-on-String Electrospun Nanofibers. J. Macromol. Sci. B 2015, 54, 887–896. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Kulkarni, A.A.; Patel, A.; Di-Luca, A.; Reis, R.L.; Gomes, M.E.; van-Blitterswijk, C.; Moroni, L.; Khademhosseini, A. Amphiphilic beads as depots for sustained drug release integrated into fibrillar scaffolds. J. Control. Release 2014, 187, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, S.; Liu, G.; Han, G.; Cheng, W.; Fu, Z.; Wu, Q.; Wang, Q. Effect of experimental parameters on morphological, mechanical and hydrophobic properties of electrospun polystyrene fibers. Materials 2015, 8, 2718–2734. [Google Scholar] [CrossRef]
- Belgharza, M.; Hassanain, I.; Lakrari, K.; El-Moudane, M.; Satrallah, A.; Elhabib, E.; El-Azzouzi, M.; Elmakhoukhi, F.; Elazzouzi, H.; El-Imache, A.; et al. Kinematic Viscosity Versus Temperature for Vegetable Oils: Argan, Avocado and Olive. Aust. J. Basic Appl. Sci. 2014, 8, 342–345. [Google Scholar]
- Siddiqui, N.; Ahmad, A. A study on viscosity, surface tension and volume flow rate of some edible and medicinal oils. Int. J. Sci. Environ. Technol. 2013, 2, 1318–1326. [Google Scholar]
- Adams, T.; Grant, C.; Watson, H. A simple algorithm to relate measured surface roughness to equivalent sand-grain roughness. Int. J. Mech. Eng. Mechatron. 2012, 1, 66–71. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Cao, X.; Zhang, S.; Wang, S.; Wu, Q. Fibrous poly(chitosan-g-dl-lactic acid) scaffolds prepared via electro-wet-spinning. Acta Biomater. 2008, 4, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Shaheen, A.; Owais, M.; Gaurav, S.S. Antimicrobial Activity of Syzygium Aromaticum Oil and its Potential in the Treatment of Urogenital Infections. Available online: http://www.formatex.info/microbiology4/vol2/865-871.pdf (accessed on 12 October 2018).
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Chahboun, N.; El Hartiti, H.; Kabouche, Z.; El M’Rabet, M.; Berrabeh, M.; Touzani, R.; Ouhssine, M.; Oudda, H. Study of the antibacterial effect of Argan oil from Bechar region of Algeria on hospital resistant strains. J. Mater. Environ. Sci. 2015, 6, 2476–2482. [Google Scholar]
- Dakiche, H.; Khali, M.; Abu-el-Haija, A.K.; Al-Maaytah, A.; Al-Balas, Q.A. Biological activities and phenolic contents of Argania spinosa L (Sapotaceae) leaf extract. Trop. J. Pharm. Res. 2016, 15, 2563–2570. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Stoleru, E.; Ioanid, E.G.; Vasile, C.; Mitelut, A.C.; Popa, M.E.; Tănase, E.E.; Mihai, A.L.; Drăghici, M.C.; Rosnes, J.T.; et al. The use of the electrospinnig technique to produce polymeric films/coatings with multifunctional properties. In Proceedings of the National Workshop “Ambalaje Alimentare Active”, Bucuresti, Romania, 28 February 2017. [Google Scholar]
- Miteluţ, A.C.; Popa, E.E.; Popescu, P.A.; Popa, M.E.; Munteanu, B.S.; Vasile, C.; Ştefănoiu, G. Research on chitosan and oil coated PLA as food packaging material. In Proceedings of the International Workshop “Progress in Antimicrobial Materials”, Iasi, Romania, 30 March 2017. [Google Scholar]
- Jing, Y.J.; Hao, Y.J.; Qu, H.; Shan, Y.; Li, D.S.; Du, R.Q. Studies on the antibacterial activities and mechanisms of chitosan obtained from cuticles of housefly larvae. Acta Biol. Hung. 2007, 58, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Kaya, M.; Asan-Ozusaglam, M.; Erdogan, S. Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J. Biosci. Bioeng. 2016, 121, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Masoud-Hosseini, S. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT-Food Sci. Technol. 2016, 71, 347–355. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, F.L. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Chang, S.H.; Lin, H.T.V.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Hongpattarakere, T.; Riyaphan, O.S. Effect of deacetylation conditions on antimicrobial activity of chitosans prepared from carapace of black tiger shrimpPenaeus monodon. J. Sci. Technol. 2008, 30, 1–9. [Google Scholar]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Andres, Y.; Giraud, L.; Gerente, C.; Le-Cloirec, P. Antibacterial effects of chitosan powder: Mechanisms of action. Environ. Technol. 2007, 12, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Youn, D.K.; Lee, S.H.; No, H.K.; Ha, J.G.; Prinyawiwatkul, W. Antibacterial activity of chitosans with different degrees of deacetylation and viscosities. Int. J. Food Sci. Technol. 2010, 45, 676–682. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Yu, Y.; Zhao, Y.; Liang, F.; Zhang, X. Strong antibacterial polydopamine coatings prepared by a shaking-assisted method. Sci. Rep. 2016, 6, 24420. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.R.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Differences in colonisation of five marine bacteria on two types of glass surfaces. Biofouling 2009, 5, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78A, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Hickey, D.J.; de-Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Barba, I.; García-Martín, J.M.; Álvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, V.K.; Webb, H.K.; Fadeeva, E.; Chichkov, B.N.; Wu, A.H.F.; Lamb, R.; Wang, J.Y.; Crawford, R.J.; Ivanova, E.P. Air-directed attachment of coccoid bacteria to the surface of superhydrophobic lotus-like titanium. Biofouling 2012, 28, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Otohinoyi, D.A.; Ekpo, O.; Ibraheem, O. Effect of ambient temperature storage on 2,2-diphenyl-1-picrylhydrazyl; DPPH as a free radical for the evaluation of antioxidant activity. Int. J. Biol. Chem. Sci. 2014, 8, 1262–1268. [Google Scholar] [CrossRef]
- Gokmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: the ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci. Food Safety 2017, 16, 382–399. [Google Scholar] [CrossRef]
- Tufan, A.N.; Celik, S.E.; Ozyurek, M.; Guclu, K.; Apak, R. Direct measurement of total antioxidant capacity of cereals: QUENCHER-CUPRAC method. Talanta 2013, 108, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of polyphenols, tocopherols, and antioxidant capacity in virgin argan oil Argania spinosa, Skeels. Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de-Souza, C.R.F.; Oliveira, W.P. Syzygium aromaticum: a precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Vasile, C.; Stoleru, E.; Irimia, A.; Zaharescu, T.; Dumitriu, R.P.; Ioanid, G.E.; Munteanu, B.S.; Oprica, L.; Pricope, G.M.; Hitruc, G.E. Ionizing Radiation And Plasma Discharge Mediating Covalent Linking of Bioactive Compounds onto Polymeric Substrate to Obtain Stratified Composites for Food Packing. In Proceedings of the the 3rd RCM of the CRP on Application of Radiation Technology in the Development of Advanced Packaging Materials for Food Products, Vienna, Austria, 11–15 July 2016. [Google Scholar]
- Vasile, C.; Pâslaru, E.; Sdrobis, A.; Pricope, G.; Ioanid, G.E.; Darie, R.N. Plasma assisted functionalization of synthetic and natural polymers to obtain new bioactive food packaging materials in Ionizing Radiation and Plasma discharge Mediating Covalent Linking of Stratified Composites Materials for Food Packaging. In Proceedings of the Part of Co-ordinated Project: ‘Application of Radiation Technology in the Development of Advanced Packaging Materials for Food Products’, Vienna, Austria, 22–26 April 2013. [Google Scholar]







| Code | Sample Description |
|---|---|
| Uncoated Samples | |
| PLA Hot-Pressed | Hot-pressed PLA films obtained from PLA 2002D pellets (NatureWorks LLC) |
| PLA-NATIVIA | Commercial NATIVIA® NTSS 40 µm PLA foils (from Taghleef Industries) |
| Coated Samples: PLA Hot-Pressed Substrate | |
| PLA-H | PLA hot-pressed film coated with Chit-H |
| HC | PLA hot-pressed film coated with Chit-H/clove oil |
| HA | PLA hot-pressed film coated with Chit-H/argan oil |
| LC | PLA hot-pressed film coated with Chit-L/clove oil |
| LA | PLA hot-pressed film coated with Chit-L/argan oil |
| Coated Samples: PLA-NATIVIA Substrate | |
| HC-NATIVIA | Commercial PLA-NATIVIA® NTSS 40 µm coated with Chit-H/clove oil |
| HA-NATIVIA | Commercial PLA-NATIVIA® NTSS 40 µm coated with Chit-H/argan oil |
| H-NATIVIA | Commercial PLA-NATIVIA® NTSS 40 µm coated with Chit-H |
| Sample | Range of the Particle Diameter Distribution (nm) | Range of the Fiber Diameter Distribution (nm) | Range of the AFM Height Distribution (nm) |
|---|---|---|---|
| HA | 20–140 | 20–140 | 150–500 |
| HC | 10–80 | 30–140 | 150–600 |
| LA | 10–60 | - | 10–55 |
| LC | 20–80 | - | 10–80 |
| Sample | Log Reduction of the Number of Viable Bacterial Cells | |
|---|---|---|
| E. coli | S. Aureus | |
| PLA Hot-Pressed | ||
| PLA (uncoated) | invalid | −0.8 |
| HC | invalid | 0.8 |
| HA | invalid | 0.7 |
| PLA-NATIVIA® NTSS 40 µm | ||
| NATIVIA (uncoated) | 1.1 | −0.7 |
| H-NATIVIA | 1.8 | 0.8 |
| HC-NATIVIA | 2.2 | 1.2 |
| HA-NATIVIA | 0.8 | 0 |
| Sample | Escherichia Coli | Listeria Monocytogenes | Salmonella Typhymurium | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Inhibition (%) | ||||||
| PLA Hot-Pressed | ||||||
| PLA -H | 26 | 42 | 29 | 32 | 22 | 29 |
| LA | 43 | 70 | 49 | 54 | 37 | 49 |
| LC | 68 | 80 | 37 | 63 | 100 | 100 |
| HA | 50 | 77 | 60 | 100 | 51 | 78 |
| HC | 80 | 91 | 77 | 100 | 100 | 100 |
| PLA NATIVIA® NTSS 40 µm 1 | ||||||
| H-NATIVIA | 10 | 53 | 16 | 58 | 35 | 71 |
| HA- NATIVIA | 49 | 82 | 47 | 100 | 55 | 94 |
| HC- NATIVIA | 53 | 78 | 53 | 100 | 65 | 90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.-L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films. Materials 2018, 11, 1973. https://doi.org/10.3390/ma11101973
Munteanu BS, Sacarescu L, Vasiliu A-L, Hitruc GE, Pricope GM, Sivertsvik M, Rosnes JT, Vasile C. Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films. Materials. 2018; 11(10):1973. https://doi.org/10.3390/ma11101973
Chicago/Turabian StyleMunteanu, Bogdanel Silvestru, Liviu Sacarescu, Ana-Lavinia Vasiliu, Gabriela Elena Hitruc, Gina M Pricope, Morten Sivertsvik, Jan Thomas Rosnes, and Cornelia Vasile. 2018. "Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films" Materials 11, no. 10: 1973. https://doi.org/10.3390/ma11101973
APA StyleMunteanu, B. S., Sacarescu, L., Vasiliu, A. -L., Hitruc, G. E., Pricope, G. M., Sivertsvik, M., Rosnes, J. T., & Vasile, C. (2018). Antioxidant/Antibacterial Electrospun Nanocoatings Applied onto PLA Films. Materials, 11(10), 1973. https://doi.org/10.3390/ma11101973