One-Pot Synthesized Visible Light-Driven BiOCl/AgCl/BiVO4 n-p Heterojunction for Photocatalytic Degradation of Pharmaceutical Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of BiOCl/AgCl/BiVO4
2.3. Characterization
2.4. Photocatalytic Activity Test
3. Results and Discussion
3.1. Structural and Chemical Characterization
3.1.1. XRD
3.1.2. SEM and TEM
3.1.3. UV-Vis Spectrophotometry
3.1.4. FTIR
3.1.5. BET
3.1.6. Raman
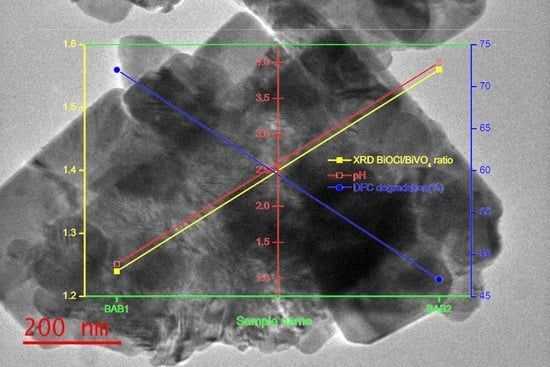
3.2. Photocatalytic Activity of BiOCl/AgCl/BiVO4 Photocatalysts
3.3. Brief Comparison of Bi-Based Photocatalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; Van Der Ploeg, M.; Van De Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Bian, Z.Y.; Zhu, Y.Q.; Zhang, J.X.; Ding, A.Z.; Wang, H. Visible-light driven degradation of ibuprofen using abundant metal-loaded BiVO4 photocatalysts. Chemosphere 2014, 117, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Zhang, D.; Pu, X.; Kong, D.; Lu, Z.; Shao, X.; Ma, H.; Dou, J. One-pot combustion synthesis of BiVO4/BiOCl composites with enhanced visible-light photocatalytic properties. Sep. Purif. Technol. 2017, 174, 97–103. [Google Scholar] [CrossRef]
- Liu, B.; Yan, X.; Yan, H.; Yao, Y.; Cai, Y.; Wei, J.; Chen, S.; Xu, X.; Li, L. Preparation and Characterization of Mo Doped in BiVO4 with Enhanced Photocatalytic Properties. Materials 2017, 10, 976. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Duan, H.; Li, H.; Sun, C.; Liu, H. Facile synthesis of V4+ self-doped, [010] oriented BiVO4 nanorods with highly efficient visible light-induced photocatalytic activity. Phys. Chem. Chem. Phys. 2014, 16, 24519–24526. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Shen, C.; Tang, G.; Zhao, S.; Zhang, J.; Chen, D.; Wei, Q.; Dong, X. Facile Construction of g-C3N4Nanosheets/TiO2Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible-Light Performance. ChemCatChem 2016, 8, 3064–3073. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Wei, Z.; Yang, S.; He, H.; Sun, C. Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4 @p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B Environ. 2016, 185, 242–252. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Noh, M.F.M.; Soh, M.F.; Tahir, A.A.; Ludin, N.A.; Ibrahim, M.A.; Isahak, W.N.R.W.; Teridi, M.A.M. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/ BiVO4 photocatalyst. Appl. Catal. B Environ. 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Tadé, M.O.; Liu, S. Construction of p-n heterojunction β-Bi2O3/BiVO4 nanocomposite with improved photoinduced charge transfer property and enhanced activity in degradation of ortho -dichlorobenzene. Appl. Catal. B Environ. 2017, 219, 259–268. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, G.; Hojamberdiev, M.; Okada, K.; Liang, J.; Luo, X.; Liu, P.; Liu, Y. Co3O4 nanoparticles-loaded BiOCl nanoplates with the dominant {001} facets: Efficient photodegradation of organic dyes under visible light. Appl. Catal. B Environ. 2014, 152, 425–436. [Google Scholar] [CrossRef]
- Meribout, R.; Zuo, Y.; Khodja, A.A.; Piram, A.; Lebarillier, S.; Cheng, J.; Wang, C.; Wong-Wah-Chung, P. Photocatalytic degradation of antiepileptic drug carbamazepine with bismuth oxychlorides (BiOCl and BiOCl/AgCl composite) in water: Efficiency evaluation and elucidation degradation pathways. J. Photochem. Photobiol. A Chem. 2016, 328, 105–113. [Google Scholar] [CrossRef]
- Huang, C.; Hu, J.; Cong, S.; Zhao, Z.; Qiu, X. Hierarchical BiOCl microflowers with improved visible-light-driven photocatalytic activity by Fe(III) modification. Appl. Catal. B Environ. 2015, 174, 105–112. [Google Scholar] [CrossRef]
- De La Garza-Galván, M.; Zambrano-Robledo, P.; Vazquez-Arenas, J.; Romero-Ibarra, I.; Ostos, C.; Peral, J.; García-Pérez, U. In situ synthesis of Au-decorated BiOCl/BiVO4 hybrid ternary system with enhanced visible-light photocatalytic behavior. Appl. Surf. Sci. 2019, 487, 743–754. [Google Scholar] [CrossRef]
- Jaihindh, D.P.; Fu, Y.P. Facile synthesis of deep eutectic solvent assisted BiOCl/BiVO4 @AgNWs plasmonic photocatalysts under visible light enhanced catalytic performance. Catal. Today 2017, 297, 246–254. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Fung, C.S.; Rather, R.A.; Lo, I.M. One-pot hydrothermal synthesis of g-C3N4/Ag/AgCl/BiVO4 micro-flower composite for the visible light degradation of ibuprofen. Chem. Eng. J. 2018, 341, 248–261. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Dongol, M.; El-Nahass, M.; El-Denglawey, A.; Elhady, A.; Abuelwafa, A.A. Optical Properties of Nano 5,10,15,20-Tetraphenyl-21H,23H-Prophyrin Nickel (II) Thin Films. Curr. Appl. Phys. 2012, 12, 1178–1184. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, D.; Lu, G.; Yan, Z.; Liu, J.; Zhou, R.; Nkoom, M. Fabrication of Fe3O4 quantum dots modified BiOCl/BiVO4 p-n heterojunction to enhance photocatalytic activity for removing broad-spectrum antibiotics under visible light. J. Taiwan Inst. Chem. Eng. 2019, 96, 681–690. [Google Scholar] [CrossRef]
- Mohammadi, R.; Esmati, S.; Gholamhosseini-Nazari, M.; Teimuri-Mofrad, R. Synthesis and characterization of a novel Fe3O4@SiO2–BenzIm-Fc[Cl]/BiOCl nano-composite and its efficient catalytic activity in the ultrasound-assisted synthesis of diverse chromene analogs. New J. Chem. 2019, 43, 135–145. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yu, J.; Leung, M.K.P.; Ho, W.; Cheng, B.; Zhao, X. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217, 69–78. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Mohseni-Bandpei, A.; Safari, A.A.; Asadi, A. N,S co-doped TiO2 nanoparticles and nanosheets in simulated solar light for photocatalytic degradation of non-steroidal anti-inflammatory drugs in water: A comparative study. J. Chem. Technol. Biotechnol. 2016, 91, 2693–2704. [Google Scholar] [CrossRef]
- Salarian, A.A.; Hami, Z.; Mirzaei, N.; Mohseni, S.M.; Asadi, A.; Bahrami, H.; Vosoughi, M.; Alinejad, A.; Zare, M.R.; Mirzaie, N.; et al. N-doped TiO2 nanosheets for photocatalytic degradation and mineralization of diazinon under simulated solar irradiation: Optimization and modeling using a response surface methodology. J. Mol. Liq. 2016, 220, 183–191. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Nitrogen and sulfur co-doped TiO2 nanosheets with exposed {001} facets: Synthesis, characterization and visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2011, 13, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, H.; Huang, H.; Liu, R.; Han, Y.; Liu, Y.; Tong, C.; Kang, Z. Carbon quantum dots enhance the photocatalytic performance of BiVO4 with different exposed facets. Dalton Trans. 2013, 42, 6285. [Google Scholar] [CrossRef]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.; Zhou, Y.; Yan, X. Deposition of silver nanoparticles onto two dimensional BiOCl nanodiscs for enhanced visible light photocatalytic and biocidal activities. RSC Adv. 2016, 6, 64911–64920. [Google Scholar] [CrossRef]
- Ning, S.; Ding, L.; Lin, Z.; Lin, Q.; Zhang, H.; Lin, H.; Long, J.; Wang, X. One-pot fabrication of Bi3O4Cl/BiOCl plate-on-plate heterojunction with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2016, 185, 203–212. [Google Scholar] [CrossRef]
- Gondal, M.A.; Chang, X.; Al-Saadi, A.A.; Yamani, Z.H.; Zhang, J.; Ji, G. BiOCl-assisted photodegradation of Rhodamine B under white light and monochromatic green pulsed laser irradiation. J. Environ. Sci. Health Part A 2012, 47, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Song, D.; Liu, H.; Qu, J. Permanganate oxidation of diclofenac: The pH-dependent reaction kinetics and a ring-opening mechanism. Chemosphere 2015, 136, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.; Liu, C.; Xu, L. Dy(III) Doped BiOCl Powder with Superior Highly Visible-Light-Driven Photocatalytic Activity for Rhodamine B Photodegradation. Nanomaterials 2018, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shi, Y.; Gao, C.; Wen, L.; Chen, J.; Song, S. BiOCl/BiVO4 p–n Heterojunction with Enhanced Photocatalytic Activity under Visible-Light Irradiation. J. Phys. Chem. C 2013, 118, 389–398. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Z.; Liao, T.; Liu, X.; Zhang, Y.; Li, L.; Li, W.; Hou, B. Preparation of BiVO4/BiOCl heterojunction photocatalyst by in-situ transformation method for norfloxacin photocatalytic degradation. J. Alloy. Compd. 2017, 702, 68–74. [Google Scholar] [CrossRef]
- Shan, L.; Liu, Y.; Suriyaprakash, J.; Ma, C.; Wu, Z.; Dong, L.; Liu, L. Highly efficient photocatalytic activities, band alignment of BiVO4/BiOCl {001} prepared by in situ chemical transformation. J. Mol. Catal. A Chem. 2016, 411, 179–187. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Wang, L.; Zhang, H.; Xu, P.; Duan, X.; Wang, S. Three-dimensional BiOI/BiOX (X = Cl or Br) nanohybrids for enhanced visible-light photocatalytic activity. Nanomaterials 2017, 7, 64. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Synthesis of Ag3PO4/G-C3N4 Composite with Enhanced Photocatalytic Performance for the Photodegradation of Diclofenac under Visible Light Irradiation. Catalysts 2018, 8, 45. [Google Scholar] [CrossRef]







| Compound | BAB1 | BAB2 | ||
|---|---|---|---|---|
| Reference Code | Score | Reference Code | Score | |
| BiOCl | 04-007-4915 | 62 | 04-007-4915 | 75 |
| BiVO4 | 04-016-0302 | 50 | 00-014-0688 | 48 |
| AgCl | 01-071-5209 | 31 | 04-007-3906 | 33 |
| Sample | SBET (m2/g) a | Pore Volume (cm3/g) b | Average Pore Size (nm) c |
|---|---|---|---|
| BAB1 | 6.16 | 0.044 | 25.9 |
| BAB2 | 5.09 | 0.033 | 23.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarzadeh, R.; Asadi, A.; Oviroh, P.O.; Jen, T.-C. One-Pot Synthesized Visible Light-Driven BiOCl/AgCl/BiVO4 n-p Heterojunction for Photocatalytic Degradation of Pharmaceutical Pollutants. Materials 2019, 12, 2297. https://doi.org/10.3390/ma12142297
Akbarzadeh R, Asadi A, Oviroh PO, Jen T-C. One-Pot Synthesized Visible Light-Driven BiOCl/AgCl/BiVO4 n-p Heterojunction for Photocatalytic Degradation of Pharmaceutical Pollutants. Materials. 2019; 12(14):2297. https://doi.org/10.3390/ma12142297
Chicago/Turabian StyleAkbarzadeh, Rokhsareh, Anvar Asadi, Peter Ozaveshe Oviroh, and Tien-Chien Jen. 2019. "One-Pot Synthesized Visible Light-Driven BiOCl/AgCl/BiVO4 n-p Heterojunction for Photocatalytic Degradation of Pharmaceutical Pollutants" Materials 12, no. 14: 2297. https://doi.org/10.3390/ma12142297
APA StyleAkbarzadeh, R., Asadi, A., Oviroh, P. O., & Jen, T. -C. (2019). One-Pot Synthesized Visible Light-Driven BiOCl/AgCl/BiVO4 n-p Heterojunction for Photocatalytic Degradation of Pharmaceutical Pollutants. Materials, 12(14), 2297. https://doi.org/10.3390/ma12142297