New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acridine Dendrimer (ACR)
2.3. Synthesis of [Cu2(ACR)(NO3)2]
2.4. Dendrimers Characterization
2.5. Cotton Fabric Functionalization with ACR and [Cu2(ACR)(NO3)2]
2.6. Cellular Toxicity
2.7. Antimicrobial Activity Test
2.8. Antibacterial Activity of Cotton Fabrics
2.9. Scanning Electron Microscopy (SEM)
2.10. Hydrophilicity of Cotton Fabrics
3. Results
3.1. Synthesis of Acridine Dendrimer
3.2. Spectral Characterizations
3.3. EPR Analysis
3.4. NMR Characterization of Dendrimer ACR
3.5. Antimicrobial Activity of ACR and [Cu2(ACR)(NO3)2]
3.6. Antibacterial Activity of Cotton Fabric
3.7. Hydrophilicity of Cotton Fabrics
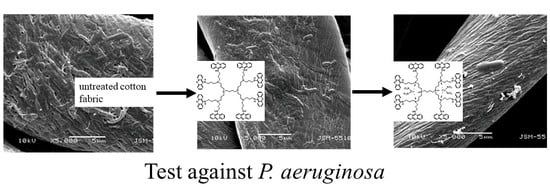
3.8. SEM Analysis
3.9. In Vitro Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Liu, S. Antibacterial surface design - Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Bin, W.; Ho, S.; Park, J.; Hak, S.; Finn, J.; Yoon, S. Discovery of torezolid as a novel 5-hydroxymethyl-oxazolidinone antibacterial agent. Eur. Med. Chem. 2011, 46, 1027–1039. [Google Scholar]
- Venepally, V.; Jala, R.C.R. An insight into the biological activities of heterocyclic-fatty acid hybrid molecules. Eur. J. Med. Chem. 2017, 141, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.H.; Addla, D.; Lv, J.S.; Zhou, C.H. Heterocyclic Naphthalimides as New Skeleton Structure of Compounds with Increasingly Expanding Relational Medicinal Applications. Curr. Top. Med. Chem. 2016, 16, 3303–3364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sahare, P.D.; Rastogi, R.C.; Ghoshal, S.K.; Mohan, D. Excited state characteristics of acridine dyes: Acriflavine and acridine orange. Spectrochim. Acta A 2003, 59, 1799–1804. [Google Scholar] [CrossRef]
- Pietrzak, M.; Szabelski, M.; Kasparek, A.; Wieczorek, Z. Interactions of hypericin with a model mutagen - Acridine orange analyzed by light absorption and fluorescence spectroscopy. Chem. Phys. Lett. 2017, 669, 85–91. [Google Scholar] [CrossRef]
- Novak, K. Chemical structures and biological activities of bis- and tetrakis-acridine derivatives: A review. J. Mol. Struct. 2017, 1146, 562–570. [Google Scholar]
- Cholewiński, G.; Dzierzbicka, K.; Kołodziejczyk, A.M. Natural and synthetic acridines/acridones as antitumor agents: Their biological activities and methods of synthesis. Pharmacol. Rep. 2011, 63, 305–336. [Google Scholar] [CrossRef]
- Palaniraja, J.; Kumar, S.S.; Ramki, S.; Arunachalam, P.; Roopan, S.M. Conventional spectroscopic identification of biologically activeimidazo-pyrimido fused acridines: In vitroanti-bacterial andanti-feedant activity. J. Mol. Liq. 2017, 230, 634–640. [Google Scholar] [CrossRef]
- Gensicka-Kowalewska, M.; Cholewinski, G.; Dzierzbicka, K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776–15804. [Google Scholar] [CrossRef] [Green Version]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P. Dendrimers and nanomedicine: Multivalency in action. New J. Chem. 2009, 33, 1809–1824. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Salgado, A.J.; Sousa, N.; Mano, J.F.; Reis, R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies. Prog. Polym. Sci. 2010, 35, 1163–1194. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Makki, M.S.I.; Sobahi, T.R.; Abdеl-Rahman, R.M.; Boyaci, I.H.; Asiri, A.M.; Grabchev, I. Synthesis and spectral characterization of a new PPA dendrimer modified with 4-bromo-1,8-naphthalimide and in vitro antimicrobial activity of its Cu(II) and Zn(II) metal complexes. Tetrahedron 2015, 71, 1080–1087. [Google Scholar] [CrossRef]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Synthesis, spectral characterization, and in vitro antimicrobial activity in liquid medium and applied on cotton fabric of a new PAMAM metallodendrimer. Int. J. Polym. Anal. Ch. 2018, 23, 45–57. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R.; Cangiotti, M.; Fattori, A.; Ottaviani, M.F. Аntimicrobial and anticancer activity of new poly (propyleneamine) metallodendrimers. J. Polym. Res. 2017, 24, 210. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Bosch, P.; Grozdanov, P.; Grabchev, I. Synthesis and Characterization of a New PAMAM Metallodendrimer for Antimicrobial Modification of Cotton Fabric. Macromol. Res. 2018, 26, 332–340. [Google Scholar] [CrossRef]
- Rahnev, I.; Rimini, G. Isotropy Equilibrium of The Double Woven Fabric with Cotton Face and Wool Reverse Fibrous Compositions. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 17th World Textile Conference (AUTEX), Corfu, Greece, 29–31 May 2017; Volume 254, pp. 675–683. [Google Scholar]
- Rahnev, I. Comparative Analysis of Single, Twisted and Sirospun Cotton. In Proceedings of the 12th World Textile Conference (AUTEX), Zadar, Yugoslavia, 13–15 June 2012; ISBN 978-2-7466-2858-8. [Google Scholar]
- Schmidt-Emrich, S.; Stiefel, P.; Rupper, P.; Katzenmeier, H.; Amberg, C.; Maniura-Weber, K.; Ren, Q. Rapid assay to assess bacterial adhesion on textiles. Materials 2016, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I. Encyclopedia of Polymer Applications; CRC Press: Boca Raton, FL, USA, 2018; pp. 2545–2562. [Google Scholar]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2000. [Google Scholar]
- Vaideki, K.; Jayakumar, S.; Rajendran, R.; Thilagavathi, G. Investigation on the effect of RF plasma and neem leaf extract treatment on the surface modification and antimicrobial activity of cotton fabric. Appl. Surf. Sci. 2008, 254, 2472–2478. [Google Scholar] [CrossRef]
- Denny, W.A.; Atwell, G.J.; Baguley, B.C.; Wakelin, L.P.G. The Acridines. J. Med. Chem. 1985, 28, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Albert, A. The Acridines, 2nd ed.; Edward Arnold Ltd.: London, UK, 1966. [Google Scholar]
- Aaron, J.J.; Maafi, M.; Párkányi, C.; Boniface, C. Quantitative treatment of the solvent effects on the electronic absorption and fluorescence spectra of acridines and phenazines. The ground and first excited singlet-state dipole moments. Spectrochim. Acta A 1995, 51, 603–615. [Google Scholar] [CrossRef]
- Ebead, Y.; Roshal, A.D.; Wróblewska, A.; Doroshenko, A.O.; Błażejowski, J. Tautomerism of acridin-9-amines substituted at the exocyclic nitrogen atom: Spectroscopic investigations and theoretical studies. Spectrochim. Acta A 2007, 66, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Karaky, K.; Chreim, Y.; Brown, R.; Blanc, S.; Lacombe, S.; Save, M.; Billon, L. From solutions to porous film: Influence of the media on fluorescent acridine endcapped polystyrene photophysic properties. Mater. Today Commun. 2015, 3, 27–35. [Google Scholar] [CrossRef]
- Medel, S.; Martínez-Campos, E.; Acitores, D.; Vasileva-Tonkova, E.; Grabchev, I.; Bosch, P. Synthesis and spectroscopic properties of a new fluorescent acridine hyperbranched polymer: Applications to acid sensing and as antimicrobial agent. Eur. Polym. J. 2018, 102, 19–29. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]
- Grabchev, I.; Bosch, P.; McKenna, M.; Nedelcheva, A. Synthesis and spectral properties of new green fluorescent poly(propyleneimine) dendrimers modified with 1,8-naphthalimide as sensors for metal cations. Polymer 2007, 48, 6755–6762. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I. Heterogeneous sensors for ammonia, amines and metal ions based on a dendrimer modified fluorescent viscose fabric. Dyes Pigments 2018, 155, 164–170. [Google Scholar] [CrossRef]
- Russell, А.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [Green Version]
- Elshaarawy, R.F.; Janiak, C. Antibacterial susceptibility of new copper (II) N-pyruvoyl anthranilate complexes against marine bacterial strains–In search of new antibiofouling candidate. Arabian J. Chem. 2016, 9, 825–834. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P.D. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. I. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F.J. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed. Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]








| Solvent | λA nm | λF nm | νA–νF cm−1 | ΦF |
|---|---|---|---|---|
| Acetonitrile | 414 | 467 | 2741 | 0.010 |
| Ethanol | 414 | 458, 481s | 2320 | 0.040 |
| Methanol | 412 | 455, 480 s | 2294 | 0.023 |
| Ethyl acetate | 392 | 467 | 4096 | 0.012 |
| Chloroform | 402 | 471 | 3644 | 0.004 |
| Tetrahydrofurane | 396 | 480 | 4420 | 0.003 |
| Dichloromethane | 408 | 469 | 3188 | 0.007 |
| Strain | MIC, µg/mL | |
|---|---|---|
| ACR | [Cu2(ACR)(NO3)2] | |
| Bacillus cereus | 40 | 10 |
| Pseudomonas aeruginosa | 120 | 80 |
| Candida lipolytica | 80 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch, P.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Kukeva, R.; Stoyanova, R.; Grabchev, I. New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials 2019, 12, 3020. https://doi.org/10.3390/ma12183020
Bosch P, Staneva D, Vasileva-Tonkova E, Grozdanov P, Nikolova I, Kukeva R, Stoyanova R, Grabchev I. New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials. 2019; 12(18):3020. https://doi.org/10.3390/ma12183020
Chicago/Turabian StyleBosch, Paula, Desislava Staneva, Evgenia Vasileva-Tonkova, Petar Grozdanov, Ivanka Nikolova, Rositsa Kukeva, Radostina Stoyanova, and Ivo Grabchev. 2019. "New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity" Materials 12, no. 18: 3020. https://doi.org/10.3390/ma12183020
APA StyleBosch, P., Staneva, D., Vasileva-Tonkova, E., Grozdanov, P., Nikolova, I., Kukeva, R., Stoyanova, R., & Grabchev, I. (2019). New Poly(Propylene Imine) Dendrimer Modified with Acridine and Its Cu(II) Complex: Synthesis, Characterization and Antimicrobial Activity. Materials, 12(18), 3020. https://doi.org/10.3390/ma12183020