Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoporous Activated Carbons
2.2. Characterizations
2.3. Electrochemical Measurements
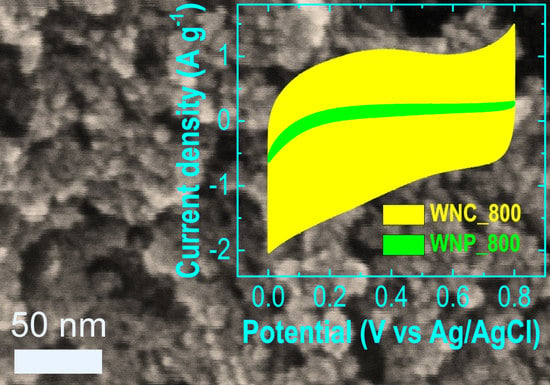
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, F.; Liu, W.; Qiu, T.; Gong, W.-B.; Ma, W.; Li, Q.; Li, F.; Geng, F. All two-dimensional pseudocapacitive sheet materials for flexible asymmetric solid-state planar microsupercapacitors with high energy density. ACS Nano 2020, 14, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chem, Z.; Yu, C.; Zhong, W. Facile synthesis of high nitrogen-doped content, mesopore-dominated biomass-derived hierarchical porous graphitic carbon for high performance supercapacitors. Electrochim. Acta 2020, 334, 135615. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El Nady, J.; Wazeer, W.; Kashyout, A.B. Development of high-performance supercapacitor based on a novel controllable green synthesis for 3D nitrogen doped graphene. Sci. Rep. 2019, 9, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Cheng, W. Recent progress in stretchable supercapacitors. J. Mater. Chem. A 2018, 6, 15478–15494. [Google Scholar] [CrossRef]
- Han, Y.; Lai, Z.; Wang, Z.; Yu, M.; Tong, Y.; Lu, X. Designing carbon based supercapacitors with high energy density: A summary of recent progress. Chem. Eur. J. 2018, 24, 7312–7329. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fua, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Vellacheri, R.; Al-Haddad, A.; Zhao, H.; Wang, W.; Wang, C.; Lei, Y. High performance supercapacitor for efficient energy storage under extreme environmental temperatures. Nano Energy 2014, 8, 231–237. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Recent progress in supercapacitors: From materials design to system construction. Adv. Mater. 2013, 25, 5336–5342. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frenzel, B.; Kurzweil, P.; Rönnebeck, H. Electromobility concept for racing cars based on lithium-ion batteries and supercapacitors. J. Power Sources 2011, 196, 5364–5376. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Yaïci, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Hannan, M.A.; Lipu, M.S.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sustain. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Xue, D. Searching for electrode materials with high electrochemical reactivity. J. Mater. 2015, 1, 170–187. [Google Scholar] [CrossRef]
- Xu, X.; Yang, T.; Zhang, Q.; Xia, W.; Ding, Z.; Eid, K.; Abdullah, A.M.; Hossain, M.S.A.; Zhang, S.; Tang, J.; et al. Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes. Chem. Eng. J. 2020, 390, 124493. [Google Scholar] [CrossRef]
- Szubzda, B.; Szmaja, A.; Halama, A. Influence of structure and wettability of supercapacitor electrodes carbon materials on their electrochemical properties in water and organic solutions. Electochim. Acta 2012, 86, 255–259. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Enviorn. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Magana, J.R.; Kolen’ko, Y.V.; Deepak, F.L.; Solans, C.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Shrestha, L.K.; Rodriguez-Abreu, C. From chromonic self-assembly to hollow carbon nanofibers: Efficient materials in supercapacitor and vapor-sensing applications. ACS Appl. Mater. Interfaces 2016, 8, 31231–31238. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Schon, T.B.; Li, H.; Fan, C.; Li, X.; Wang, H.; Wu, X.; Xie, H.; Sun, H.; et al. Porous carbon with Willow-leaf-shaped pores for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 42699–42707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, M.; Zhang, Y.; Zhao, W.; Wang, C. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D mesoporous carbon microbelts derived from fullerene crystals as an electrode material for electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465. [Google Scholar] [CrossRef] [PubMed]
- Bairi, P.; Shrestha, R.G.; Hill, J.P.; Nishimura, T.; Ariga, K.; Shrestha, L.K. Mesoporous graphitic carbon microtubes derived from fullerene C70 tubes as a high performance electrode material for advanced supercapacitors. J. Mater. Chem. A 2016, 4, 13899–13906. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, Z.; Gao, Y.; An, W.; Cao, Z.; Liu, J. Biomass-swelling assisted synthesis of hierarchical porous carbon fibers for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28283–28290. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for metal−organic framework-derived nanoporous carbons toward supercapacitor applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Adhikari, L.; Shrestha, R.G.; Adhikari, M.P.; Adhikari, R.; Hill, J.P.; Pradhananga, R.R.; Ariga, K. Nanoporous carbon materials with enhanced supercapacitance performance and non-aromatic chemical sensing with C1/C2 alcohol discrimination. Sci. Technol. Adv. Mater. 2016, 17, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macroporous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Sun, G.; Su, F.; Kong, Q.-Q.; Li, F.; Ma, W.; Shi, J.; Jiang, D.; Lu, C.; et al. Hollow carbon microtubes from kapok fiber: Structural evolution and energy storage performance. Sustain. Energy Fuels 2018, 2, 455–465. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, H.; Wang, H.; Zhao, X.; Sun, S.; Shi, J.; Huang, M.; Liu, W.; Zheng, Y.; Li, P. Dual-doped hierarchical porous carbon derived from biomass for advanced supercapacitors and lithium ion batteries. RSC Adv. 2019, 9, 32382–32394. [Google Scholar] [CrossRef] [Green Version]
- Kaipannan, S.; Marappan, S. Fabrication of 9.6 V high-performance asymmetric supercapacitors stack based on nickel hexacyanoferrate-derived Ni(OH)2 nanosheets and bio-derived activated carbon. Sci. Rep. 2019, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Pan, M.; Feng, Z.; Qin, Y.; Wang, Y.; Tan, L.; Sun, T. Ultra-high adsorption of tetracycline antibiotics on garlic skin-derived porous biomass carbon with high surface area. New J. Chem. 2020, 44, 1097–1106. [Google Scholar] [CrossRef]
- Ghosh, S.; Santhosh, R.; Jeniffer, S.; Raghavan, V.; Jacob, G.; Nanaji, K.; Kollu, P.; Jeong, S.K.; Grace, A.N. Natural biomass derived hard carbon and activated carbons as electrochemical supercapacitor electrodes. Sci. Rep. 2019, 9, 16315. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, L.K.; Thapa, M.; Shrestha, R.G.; Maji, S.; Pradhananga, R.R.; Ariga, K. Rice husk-derived high surface area nanoporous carbon materials with excellent iodine and methylene blue adsorption properties. C J. Carbon Res. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Genovese, M.; Jiang, J.; Lian, K.; Holmb, N. High capacitive performance of exfoliated biochar nanosheets from biomass saste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. Biowaste-derived 3D honeycomb-like porous carbon with binary-heteroatom doping for high-performance flexible solid-state supercapacitors. J. Mater. Chem. A 2018, 6, 160–166. [Google Scholar] [CrossRef]
- Niksiar, A.; Nasernejad, B. Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass Bioenergy 2017, 106, 43–50. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q. Water bamboo-derived porous carbons as electrode materials for supercapacitors. New J. Chem. 2015, 39, 3859–3864. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Gao, S. Biomass-derived interconnected carbon nanoring electrochemical capacitors with high performance in both strongly acidic and alkaline electrolytes. J. Mater. Chem. A 2017, 5, 181–188. [Google Scholar] [CrossRef]
- Yuan, G.; Huang, W.; Guan, K.; Li, H.; Xie, Y.; Liang, Y.; Liu, Y.; Zheng, M. A universal KOH-free strategy towards nitrogen doped carbon nanosheets for high-rate and high energy storage devices. J. Mater. Chem. A 2019, 7, 26469–26478. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shrestha, R.G.; Maji, S.; Pokahrel, B.P.; Rajbhandari, R.; Shrestha, R.L.; Pradhananga, R.R.; Hill, J.P.; Ariga, K. High surface area nanoporous graphitic carbon materials derived from Lapsi seed with enhanced supercapacitance. Nanomaterials 2020, 10, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Wang, J.; Shrestha, L.K.; Hossain, S.A.; Alothman, Z.A.; Yamauchi, Y.; Ariga, K. Activated Porous Carbon Spheres with Customized Mesopores through Assembly of Diblock Copolymers for Electrochemical Capacitor. ACS Appl. Mater. Interfaces 2017, 9, 18986–18993. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Maji, S.; Shrestha, L.K.; Ariga, K. Nanoarchitectonics of nanoporous carbon materials in supercapacitors applications. Nanomaterials 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesas, R.H.; Arami-Niya, A.; Daud, W.M.A.W.; Sahu, J.N. Preparation and characterization of activated carbon from apple waste by microwave-assisted phosphoric acid activation: Application in methylene blue adsorption. BioResources 2013, 8, 2950–2966. [Google Scholar]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Hirst, E.A.; Taylor, A.; Mokaya, R. A simple flash carbonization route for conversion of biomass to porous carbons with high CO2 storage capacity. J. Mater. Chem. A 2018, 6, 12393–12403. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Briggs, M.E.; Hu, C.-C.; Cooper, A.I. Controlling electric double-layer capacitance and pseudocapacitance in heteroatom-doped carbons derived from hypercrosslinked microporous polymers. Nano Energy 2018, 46, 277–289. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhou, L.; Zhang, Y.; Sun, J.; Wen, J.; Yuan, Y. Upgrading earth-abundant biomass into three dimensional carbon materials for energy and environmental applications. J. Mater. Chem. A 2019, 7, 4217–4229. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Gutiérrez-Pardo, A.; Ramírez-Rico, J.; Cabezas-Rodríguez, R.; Martínez-Fernández, J. Effect of catalytic graphitization on the electrochemical behavior of wood derived carbons for use in supercapacitors. J. Power Sources 2015, 278, 18–26. [Google Scholar] [CrossRef] [Green Version]






| Carbon Sample | SSA (m2 g−1) | Smicro (m2 g−1) | Smeso (m2 g−1) | Vp (cm3 g−1) | Vmicro (cm3 g−1) | Dmeso (nm) | Dmicro (nm) |
|---|---|---|---|---|---|---|---|
| WNP_800 | 39.2 | 15.3 | 23.9 | 0.099 | 0.037 | 3.09 | − |
| WNC_400 | 922.4 | 836.5 | 85.9 | 0.577 | 0.444 | 3.88 | 0.573 |
| WNC_600 | 1157.6 | 1080.5 | 77.1 | 0.662 | 0.535 | 3.88 | 0.548 |
| WNC_800 | 1309.8 | 1196.1 | 113.7 | 0.798 | 0.618 | 3.88 | 0.599 |
| WNC_1000 | 1170.3 | 1045.9 | 124.4 | 0.786 | 0.601 | 3.88 | 0.573 |
| Biomass | Electrolyte | Current Density/Scan Rate | Specific Capacitance (F g−1) | Reference |
|---|---|---|---|---|
| Washnut | 1 M H2SO4 | 1 A g−1 | 225.1 | This work |
| Bio-decomposited product (Humic acids) | 6 M KOH | 0.05 A g−1 | 209 | [26] |
| Cotton | 3 M KOH | 0.3 A g−1 | 221.7 | [29] |
| Bamboo | 1 M H2SO4 | 5 mV s−1 | 256 | [31] |
| Corn cob | 0.5 M H2SO4 | 0.5 A g−1 | 210 | [39] |
| Lapsi seed | 1 M H2SO4 | 1 A g−1 | 284 | [46] |
| Beech (Fagus sylvatica) | 1 M KOH | 20 mA g−1 | 133 | [56] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance. Materials 2020, 13, 2371. https://doi.org/10.3390/ma13102371
Shrestha RL, Shrestha T, Tamrakar BM, Shrestha RG, Maji S, Ariga K, Shrestha LK. Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance. Materials. 2020; 13(10):2371. https://doi.org/10.3390/ma13102371
Chicago/Turabian StyleShrestha, Ram Lal, Timila Shrestha, Birendra Man Tamrakar, Rekha Goswami Shrestha, Subrata Maji, Katsuhiko Ariga, and Lok Kumar Shrestha. 2020. "Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance" Materials 13, no. 10: 2371. https://doi.org/10.3390/ma13102371
APA StyleShrestha, R. L., Shrestha, T., Tamrakar, B. M., Shrestha, R. G., Maji, S., Ariga, K., & Shrestha, L. K. (2020). Nanoporous Carbon Materials Derived from Washnut Seed with Enhanced Supercapacitance. Materials, 13(10), 2371. https://doi.org/10.3390/ma13102371