Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Specimen Preparation
2.2. Microstructure Examination
2.3. Depth Profile Analysis
2.4. X-ray Photoelectron Spectroscopy Analysis
2.5. Electrochemical Measurements
2.6. Cyclic Corrosion Test for ED Coated Samples
3. Results and Discussion
3.1. Microstructure Characterizations of Metallic Coatings
3.2. Electrochemical Characterizations
3.3. Characterization of the Corrosion Products by XPS
3.4. Surface Characteristics after Electropainting
3.5. Corrosion Resistance Evaluations after Electropainting
4. Conclusions
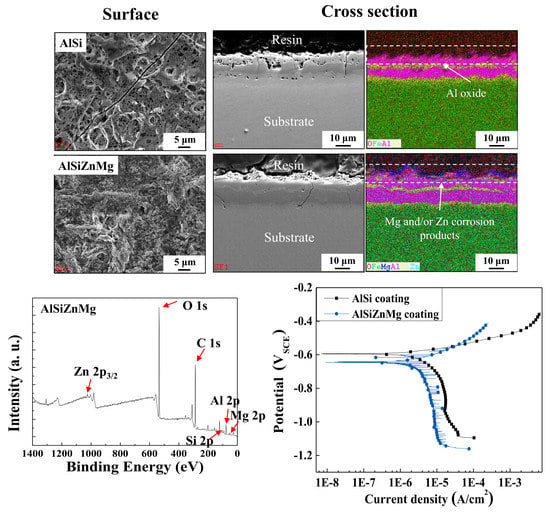
- The two types of AlSi-based coating (AlSi-MC and AlSiZnMg-MC) were composed mainly of an Al matrix with two (Al,Fe,Si)-rich intermetallic phases. The compositional features were different in that the intermetallic phases of MgZn2 and Mg2Si existed only in AlSiZnMg-MC covered with a thin outermost layer of (Zn, Mg)-based oxide.
- The active phases formed in AlSiZnMg-MC, such as Mg2Si and MgZn2, can decrease the corrosion potential. The preferential dissolution of Mg and Zn from the active phases can lead to rapid coverage of the coating surface by the precipitation of corrosion products with a protective nature, resulting in a much smaller current density for both anodic and cathodic reactions. On the other hand, the polarization curve of AlSiZnMg-MC showed many fluctuations, which may be closely associated with localized corrosion caused by the non-uniform distribution of MgO or the selective dissolution of Mg from Mg2Si phases in the coating.
- Compared to the case of the AlSi-MC, XPS showed that the surface of AlSiZnMg-MC was composed of a wider variety of corrosion products, including Al2O3, Mg(OH)2, SiO2, Zn(OH)2, Zn5(OH)8Cl2·H2O, and Mg-Al LDH. Among the products, the presence of Zn5(OH)8Cl2·H2O and Mg-Al LDH, stabilized by Mg(OH)2, is the major mechanistic reason for the higher corrosion resistance of AlSiZnMg-MC.
- In contrast to the corrosion resistance of metallic coatings, ED-coated AlSiZnMg-MC exhibited more severe surface degradation after the accelerated corrosion tests. The non-uniform formation of Mg-based oxide over the AlSiZnMg-MC can weaken the adhesion between the inner metallic coating/outer ED coating. Hence, the corrosive species are more able to permeate through the ED coating on AlSiZnMg-MC, and the electrochemical corrosion reactions occurred rapidly. Therefore, further optimization of the alloy contents in AlSi-based coatings needs to be investigated.
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control, 2nd ed.; Butterworth-Heinemann: London, UK, 2006. [Google Scholar]
- Forsyth, M.; Hinton, B. Rare Earth-Based Corrosion Inhibitors; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Schürz, S.; Luckeneder, G.H.; Fleischanderl, M.; Mack, P.; Gsaller, H.; Kneissl, A.C.; Mori, G. Chemistry of corrosion products on Zn-Al-Mg alloy coated steel. Corros. Sci. 2010, 52, 3271–3279. [Google Scholar] [CrossRef]
- Thebault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Investigation of self-healing mechanism on galvanized steels cut edges by coupling SVET and numerical modeling. Electrochim. Acta 2008, 53, 5226–5234. [Google Scholar] [CrossRef]
- Ogle, K.; Morel, S.; Jacquet, D. Observation of self-healing functions on the cut edge of galvanized steel using SVET and pH microscopy. J. Electrochem. Soc. 2006, 153, B1–B5. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, Z.; Li, C.; Tan, X.; Zhang, Z. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Zhu, X.M. Electrochemical polarization and passive film analysis of austenitic Fe–Mn–Al steels in aqueous solutions. Corros. Sci. 1999, 41, 1817–1833. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Singh Raman, R.K.; Khoddam, S. Comparative studies on the corrosion properties of a Fe–Mn–Al–Si steel and an interstitial-free steel. Corros. Sci. 2008, 50, 2879–2884. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminum and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Wang, S.; Jiang, L.; Liu, X. Influence of silicon on hot-dip aluminizing process and subsequent oxidation for preparing hydrogen/tritium permeation barrier. Int. J. Hydrog. Energy 2010, 35, 2689–2693. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. Microstructural evolution of intermetallic layer in hot-dipped aluminide mild steel with silicon addition. Surf. Coat. Technol. 2011, 205, 4726–4731. [Google Scholar] [CrossRef]
- Allely, C.; Dosdat, L.; Clauzeau, O.; Ogle, K.; Volovitch, P. Anticorrosion mechanisms of aluminized steel for hot stamping. Surf. Coat. Technol. 2014, 238, 188–196. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of Al. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium; Elsevier: Oxford, UK, 2004. [Google Scholar]
- Salgueiro Azevedo, M.; Allely, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn (Mg,Al) coated steel: The effect of HCO3− and NH4+ ions on the intrinsic reactivity of the coating. Electrochim. Acta 2015, 153, 159–169. [Google Scholar] [CrossRef]
- Volovitch, P.; Vu, T.N.; Allély, C.; Abdel Aal, A.; Ogle, K. Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn–Al–Mg coatings on steel. Corros. Sci. 2011, 53, 2437–2445. [Google Scholar] [CrossRef]
- Prosek, T.; Larché, N.; Vlot, M.; Goodwin, F.; Thierry, D. Corrosion performance of Zn–Al–Mg coatings in open and confined zones in conditions simulating automotive applications. Mater. Corros. 2010, 61, 412–420. [Google Scholar] [CrossRef]
- Nicarda, C.; Allély, C.; Volovitch, P. Effect of Zn and Mg alloying on microstructure and anticorrosion mechanisms of Al-Si based coatings for high strength steel. Corros. Sci. 2019, 146, 192–201. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, C.W.; Shin, G.Y.; Yoo, J.H.; Choi, M. Study of the corrosion behavior, liquid metal embrittlement and resistance spot weldability of galvannealed hot stamping steel. Korean J. Met. Mater. 2019, 57, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Kharitonov, D.S.; Örnek, C.; Claesson, P.M.; Sommertune, J.; Zharskii, I.M.; Kurilo, I.I.; Pan, J. Corrosion inhibition of aluminum alloy AA6063-T5 by vanadates, microstructure characterization and corrosion analysis. J. Electrochem. Soc. 2018, 165, C116–C126. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Ahn, S.H.; Kim, S.J. Effects of Zn and Mg addition to AlSi metallic coating for hot stamping steel. In Hyundai Steel Technical Report; Hyundai Steel Company: Chungnam, Korea, 2019; Volume 7, pp. 12–17. ISSN 2288-6990. [Google Scholar]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments; ASTM D1654–08; ASTM International: West Conshohocken, PA, USA, 2016.
- Standard Test Method for Chipping Resistance of Coatings; ASTM D3170; ASTM International: West Conshohocken, PA, USA, 2014.
- Wang, C.J.; Chen, S.M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601–6605. [Google Scholar] [CrossRef]
- Jo, K.R.; Cho, L.; Sulistiyou, D.H.; Seo, E.J.; Kim, S.W.; De Cooman, B.C. Effects of Al-Si coating and Zn coating on the hydrogen uptake and embrittlement of ultra-high strength press-hardened steel. Surf. Coat. Technol. 2019, 374, 1108–1119. [Google Scholar] [CrossRef]
- Grigorieva, R.; Drillet, P.; Mataigne, J.M.; Redjaïmia, A. Phase transformations in the Al-Si coating during the austenitization step. Solid State Phenom. 2011, 172–174, 784–790. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic compound formation in Fe–Al–Si ternary system: Part II. Mater. Charact. 2003, 49, 293–311. [Google Scholar] [CrossRef]
- Bruschi, S.; Ghiotti, A. Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Windmann, M.; Röttger, A.; Theisen, W. Phase formation at the interface between a boron alloyed steel substrate and an Al-rich coating. Surf. Coat. Technol. 2013, 226, 130–139. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yakou, T. Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment. Mater. Sci. Eng. A 2002, 338, 44–53. [Google Scholar] [CrossRef]
- Cho, Y.R.; Kim, T.H.; Park, S.H.; Baek, E.R. Aluminum-Plated Steel Sheet Having Superior Corrosion Resistance, Hot Press Formed Product Using the Same, and Method for Production Thereof. European Patent WO/2010/079995, 15 July 2010. [Google Scholar]
- Li, Q.; Zhao, Y.Z.; Luo, Q.; Chen, S.L.; Zhang, J.Y.; Chou, K.C. Experimental study and phase diagram calculation in Al–Zn–Mg–Si quaternary system. J. Alloy. Compd. 2010, 501, 282–290. [Google Scholar] [CrossRef]
- Samsonov, G.V. The Oxide Handbook, 2nd ed.; IFI/Plenum: New York, NY, USA, 1973. [Google Scholar]
- Lee, J.W.; Park, B.R.; Oh, S.Y.; Yun, D.W.; Hwang, J.K.; Oh, M.S.; Kim, S.J. Mechanistic study on the cut-edge corrosion behaviors of Zn-Al-Mg alloy coated steel sheets in chloride containing environments. Corros. Sci. 2019, 160, 108170. [Google Scholar] [CrossRef]
- Volovitch, P.; Barrallier, L.; Saikaly, W. Microstructure and corrosion resistance of Mg alloy ZE41 with laser surface cladding by Al–Si powder. Surf. Coat. Technol. 2008, 202, 4901–4914. [Google Scholar] [CrossRef]
- Salgueiro Azevedo, M.; Allely, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn (Mg,Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Hosking, N.C.; StrÖm, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc–magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Ishizaki, T.; Kamiyama, N.; Watanabe, K.; Serizawa, A. Corrosion resistance of Mg(OH)2/Mg–Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Corros. Sci. 2015, 92, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Pan, F.; Liu, Y.; Zhang, G.; Tang, A.; Atrens, A. Influence of pH on the growth behavior of Mg–Al LDH films. Surf. Eng. 2017, 34, 674–681. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.L.; Song, L.; Zeng, R.C.; Liu, Z.G.; Cui, H.Z. Corrosion of in-situ grown MgAl-LDH coating on aluminum alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.O.; Yang, W.S.; Kim, S.J. Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets. Materials 2020, 13, 3379. https://doi.org/10.3390/ma13153379
Kim SO, Yang WS, Kim SJ. Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets. Materials. 2020; 13(15):3379. https://doi.org/10.3390/ma13153379
Chicago/Turabian StyleKim, Si On, Won Seog Yang, and Sung Jin Kim. 2020. "Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets" Materials 13, no. 15: 3379. https://doi.org/10.3390/ma13153379
APA StyleKim, S. O., Yang, W. S., & Kim, S. J. (2020). Effects of the Combined Addition of Zn and Mg on Corrosion Behaviors of Electropainted AlSi-Based Metallic Coatings Used for Hot-Stamping Steel Sheets. Materials, 13(15), 3379. https://doi.org/10.3390/ma13153379