Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chamotte
2.1.2. Biomass Bottom Ash from the Combustion of Almond Husk and Alpeorujo
2.2. Methodology
2.2.1. Initial Tests of the by-Products
2.2.2. Conformed of Geopolymers: Physical and Mechanical Tests of the Conformed Samples
- The chamotte and the biomass bottom ash were mixed until the resulting mass was homogenized and according to the corresponding percentages of each family.
- Subsequently, 20% water was added to the previous mass, mixing again until obtaining the homogenization of the product.
- This resulting mixture was conformed in a steel matrix of internal dimensions of 60 × 30 mm, applying a gradual pressure through a piston until reaching 30 ± 1 MPa. This pressure was maintained for one minute.
- Once the mixture was compacted, the sample was removed, leaving the sample fully conformed.
2.2.3. Ageing Tests (Freezing Test) and Fourier Transform Infrared (FTIR) of the Geopolymers
3. Results and Discussions
3.1. Initial Tests of by-Products
3.2. Physical and Mechanical Tests of the Conformed Samples
3.3. Ageing Tests (Freezing Test) and Fourier Transform Infrared (FTIR) of the Geopolymers
4. Conclusions
- The physical–chemical characterization of the chamotte and the biomass bottom ashes showed the suitability of both materials for the conformation of geopolymers. The elemental composition of the chamotte provides the perfect base of aluminosilicate, in combination with the high percentage of potassium present in the biomass bottom ashes. On the other hand, the similarity between the densities of both by-products and their microscopic granulometry facilitates the mixing process.
- The physical tests carried out on the families of samples conformed have reflected logical and statistically representative behavior. The loss of weight and linear shrinkage increased as the percentage of BBA in the mix increased. However, the bulk density is much lower than that of a traditional ceramic, which is of interest for other properties such as thermal or acoustic insulation. On the other hand, the rate of capillary water absorption, the cold water absorption and boiling water absorption, as well as the open porosity, decreased as the percentage of BBA in the mixture increased.
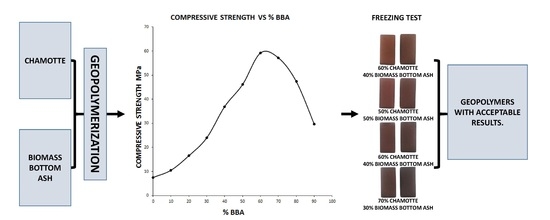
- The mechanical tests reflected a perfect quadratic curve, with a maximum of around 60% biomass bottom ashes in the mixture. However, all the families, except for 10C0A and 0C10A, showed adequate resistance behavior according to the regulations in force.
- The freezing tests determined that only the 6C4A, 5C5A, 4C6A and 3C7A families have adequate resistance to the ageing test.
- The Fourier transform infrared (FTIR) analysis reflected the formation of the geopolymer for the 6C4A, 5C5A, 4C6A and 3C7A sample groups.
- Geopolymers with acceptable results are formed with 40% BBA and 60% chamotte up to 70% BBA and 30% chamotte, the optimum combination being 60% BBA and 40% chamotte.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Liu, B.; Du, J.; Liu, C.; Wang, S. CO2 emission linkage analysis in global construction sectors: Alarming trends from 1995 to 2009 and possible repercussions. J. Clean. Prod. 2019, 221, 863–877. [Google Scholar] [CrossRef]
- Oti, J.E.; Kinuthia, J.M. Stabilised unfired clay bricks for environmental and sustainable use. Appl. Clay Sci. 2012, 58, 52–59. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Munir, M.J.; Khitab, A. Exploratory study on the effect of waste rice husk and sugarcane bagasse ashes in burnt clay bricks. J. Build. Eng. 2016, 7, 372–378. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41. [Google Scholar] [CrossRef]
- Subashi De Silva, G.H.M.J.; Mallwattha, M.P.D. Strength, durability, thermal and run-off properties of fired clay roof tiles incorporated with ceramic sludge. Constr. Build. Mater. 2018, 179, 390–399. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sui, T. Alternative fuels—Effects on clinker process and properties. Cem. Concr. Res. 2019, 123, 105777. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Sun, J.; Wang, D.; Liu, C.; Luther, M.; Xu, Y. Composition of energy outflows embodied in the gross exports of the construction sector. J. Clean. Prod. 2020, 248, 119296. [Google Scholar] [CrossRef]
- Dondi, M.; Guarini, G.; Raimondo, M.; Zanelli, C. Recycling PC and TV waste glass in clay bricks and roof tiles. Waste Manag. 2009, 29, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.J.; Kazmi, S.M.S.; Wu, Y.F.; Hanif, A.; Khan, M.U.A. Thermally efficient fired clay bricks incorporating waste marble sludge: An industrial-scale study. J. Clean. Prod. 2018, 174, 1122–1135. [Google Scholar] [CrossRef]
- Thapa, V.B.; Waldmann, D.; Wagner, J.F.; Lecomte, A. Assessment of the suitability of gravel wash mud as raw material for the synthesis of an alkali-activated binder. Appl. Clay Sci. 2018, 161, 110–118. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Cazacliu, B.G.; Cothenet, A.; Poullain, P.; Wilhelm, M.; Sampaio, C.H.; Lima, E.C.; Ambros, W.; Torrenti, J.M. Fabrication, microstructure, and properties of fired clay bricks using construction and demolition waste sludge as the main additive. J. Clean. Prod. 2020, 258, 120733. [Google Scholar] [CrossRef]
- Raut, S.P.; Ralegaonkar, R.V.; Mandavgane, S.A. Development of sustainable construction material using industrial and agricultural solid waste: A review of waste-create bricks. Constr. Build. Mater. 2011, 25, 4037–4042. [Google Scholar] [CrossRef]
- Saboya, F.; Xavier, G.C.; Alexandre, J. The use of the powder marble by-product to enhance the properties of brick ceramic. Constr. Build. Mater. 2007, 21, 1950–1960. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, C.; Duan, P.; Liu, Y.; Zhang, Z.; Qiu, X.; Li, D. A comparative study of high- and low-Al2O3 fly ash based-geopolymers: The role of mix proportion factors and curing temperature. Mater. Des. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Gharzouni, A.; Vidal, L.; Essaidi, N.; Joussein, E.; Rossignol, S. Recycling of geopolymer waste: Influence on geopolymer formation and mechanical properties. Mater. Des. 2016, 94, 221–229. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Yang, T.; Li, L.; Zhu, H.; Wang, H. Conversion of local industrial wastes into greener cement through geopolymer technology: A case study of high-magnesium nickel slag. J. Clean. Prod. 2017, 141, 463–471. [Google Scholar] [CrossRef]
- Shang, J.; Dai, J.G.; Zhao, T.J.; Guo, S.Y.; Zhang, P.; Mu, B. Alternation of traditional cement mortars using fly ash-based geopolymer mortars modified by slag. J. Clean. Prod. 2018, 203, 746–756. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Luukkonen, T.; Kinnunen, P.; Illikainen, M. One-part geopolymer cement from slag and pretreated paper sludge. J. Clean. Prod. 2018, 185, 168–175. [Google Scholar] [CrossRef]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A. Chloride ingress and steel corrosion in geopolymer concrete based on long term tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Sabbatini, A.; Vidal, L.; Pettinari, C.; Sobrados, I.; Rossignol, S. Control of shaping and thermal resistance of metakaolin-based geopolymers. Mater. Des. 2017, 116, 374–385. [Google Scholar] [CrossRef]
- Shi, C.; Jiménez, A.F. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Zhang, M.; Deskins, N.A.; Zhang, G.; Cygan, R.T.; Tao, M. Modeling the Polymerization Process for Geopolymer Synthesis through Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2018, 122, 6760–6773. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Li, X.; Tan, J.; Vandevyvere, B. Fly ash-based geopolymer with self-heating capacity for accelerated curing. J. Clean. Prod. 2020, 261, 121119. [Google Scholar] [CrossRef]
- Qian, L.-P.; Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Experimental study on full-volume fly ash geopolymer mortars: Sintered fly ash versus sand as fine aggregates. J. Clean. Prod. 2020, 263, 121445. [Google Scholar] [CrossRef]
- Chuah, S.; Duan, W.H.; Pan, Z.; Hunter, E.; Korayem, A.H.; Zhao, X.L.; Collins, F.; Sanjayan, J.G. The properties of fly ash based geopolymer mortars made with dune sand. Mater. Des. 2016, 92, 571–578. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F. Using fly ash to partially substitute metakaolin in geopolymer synthesis. Appl. Clay Sci. 2014, 88–89, 194–201. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud)—Putting things in perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Nazari, A.; Sanjayan, J.G. Synthesis of geopolymer from industrial wastes. J. Clean. Prod. 2015, 99, 297–304. [Google Scholar] [CrossRef]
- Pontikes, Y.; Machiels, L.; Onisei, S.; Pandelaers, L.; Geysen, D.; Jones, P.T.; Blanpain, B. Slags with a high Al and Fe content as precursors for inorganic polymers. Appl. Clay Sci. 2013, 73, 93–102. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Manzi, S.; Lancellotti, I.; Kamseu, E.; Barbieri, L.; Leonelli, C. Mix-design and characterization of alkali activated materials based on metakaolin and ladle slag. Appl. Clay Sci. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q.; Du, J. Investigation of the waterproof property of alkali-activated metakaolin geopolymer added with rice husk ash. J. Clean. Prod. 2019, 230, 603–612. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2020, 244, 118844. [Google Scholar] [CrossRef]
- Kuenzel, C.; Neville, T.P.; Donatello, S.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83–84, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Si, R.; Dai, Q.; Guo, S.; Wang, J. Mechanical property, nanopore structure and drying shrinkage of metakaolin-based geopolymer with waste glass powder. J. Clean. Prod. 2020, 242, 118502. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J.A. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef]
- Faisal, M.; Muhammad, K. Synthesis and characterization of geopolymer from bagasse bottom ash, waste of sugar industries and naturally available china clay. J. Clean. Prod. 2016, 129, 491–495. [Google Scholar]
- Arulrajah, A.; Kua, T.A.; Suksiripattanapong, C.; Horpibulsuk, S.; Shen, J.S. Compressive strength and microstructural properties of spent coffee grounds-bagasse ash based geopolymers with slag supplements. J. Clean. Prod. 2017, 162, 1491–1501. [Google Scholar] [CrossRef]
- Nkwaju, R.Y.; Djobo, J.N.Y.; Nouping, J.N.F.; Huisken, P.W.M.; Deutou, J.G.N.; Courard, L. Iron-rich laterite-bagasse fibers based geopolymer composite: Mechanical, durability and insulating properties. Appl. Clay Sci. 2019, 183, 105333. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Fu, S.; He, P.; Wang, M.; Cui, J.; Wang, M.; Duan, X.; Yang, Z.; Jia, D.; Zhou, Y. Hydrothermal synthesis of pollucite from metakaolin-based geopolymer for hazardous wastes storage. J. Clean. Prod. 2020, 248, 119240. [Google Scholar] [CrossRef]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Reaction kinetics of fly ash geopolymerization: Role of particle size controlled by using ball mill. Adv. Powder Technol. 2019, 30, 1079–1088. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Tan, K.H.; Yang, E.-H. Effect of alkali cation type on strength endurance of fly ash geopolymers subject to high temperature exposure. Mater. Des. 2018, 154, 8–19. [Google Scholar] [CrossRef]
- Part, W.K.; Ramli, M.; Cheah, C.B. An overview on the influence of various factors on the properties of geopolymer concrete derived from industrial by-products. Constr. Build. Mater. 2015, 77, 370–395. [Google Scholar] [CrossRef]
- Peyne, J.; Gautron, J.; Doudeau, J.; Rossignol, S. Development of low temperature lightweight geopolymer aggregate, from industrial Waste, in comparison with high temperature processed aggregates. J. Clean. Prod. 2018, 189, 47–58. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Sékou, T.; Siné, D.; Lanciné, T.D.; Bakaridjan, C. Synthesis and Characterization of a Red Mud and Rice Husk Based Geopolymer for Engineering Applications. Macromol. Symp. 2017, 373, 1600090. [Google Scholar] [CrossRef]
- Huiskes, D.M.A.; Keulen, A.; Yu, Q.L.; Brouwers, H.J.H. Design and performance evaluation of ultra-lightweight geopolymer concrete. Mater. Des. 2016, 89, 516–526. [Google Scholar] [CrossRef]
- Hu, Z.; Wyrzykowski, M.; Lura, P. Estimation of reaction kinetics of geopolymers at early ages. Cem. Concr. Res. 2020, 129, 105971. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Sellami, M.; Barre, M.; Toumi, M. Synthesis, thermal properties and electrical conductivity of phosphoric acid-based geopolymer with metakaolin. Appl. Clay Sci. 2019, 180, 105192. [Google Scholar] [CrossRef]
- Bi, S.; Liu, M.; Shen, J.; Hu, X.M.; Zhang, L. Ultrahigh Self-Sensing Performance of Geopolymer Nanocomposites via Unique Interface Engineering. ACS Appl. Mater. Interfaces 2017, 9, 12851–12858. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; de Gutiérrez, R.M. A novel geopolymer application: Coatings to protect reinforced concrete against corrosion. Appl. Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Yan, S.; He, P.; Jia, D.; Duan, X.; Yang, Z.; Wang, S.; Zhou, Y. In-situ preparation of fully stabilized graphene/cubic-leucite composite through graphene oxide/geopolymer. Mater. Des. 2016, 101, 301–308. [Google Scholar] [CrossRef]
- Yan, D.; Chen, S.; Zeng, Q.; Xu, S.; Li, H. Correlating the elastic properties of metakaolin-based geopolymer with its composition. Mater. Des. 2016, 95, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mazzocchi, M.; Laghi, L.; Morganti, M.; Francisconi, J.; Landi, E. Production and characterization of lightweight vermiculite/geopolymer-based panels. Mater. Des. 2015, 85, 266–274. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; França, B.R.; Alexandre, J.; Marvila, M.T.; Zanelato, E.B.; Xavier, G.C. Influence of sintering temperature of a ceramic substrate in mortar adhesion for civil construction. J. Build. Eng. 2018, 19, 342–348. [Google Scholar] [CrossRef]
- Kittl, P.; Diaz, G.; Alarcón, H. Dosification of a cement-talc-chamotte refractory mortar subjected to thermal shock. Cem. Concr. Res. 1992, 22, 736–742. [Google Scholar] [CrossRef]
- Fiala, L.; Konrád, P.; Fořt, J.; Keppert, M.; Černý, R. Application of ceramic waste in brick blocks with enhanced acoustic properties. J. Clean. Prod. 2020, 261, 121185. [Google Scholar] [CrossRef]
- Nayana, A.M.; Rakesh, P. Strength and durability study on cement mortar with ceramic waste and micro-silica. Mater. Today Proc. 2018, 5, 24780–24791. [Google Scholar] [CrossRef]
- Amin, S.K.; El–Sherbiny, S.A.; El–Magd, A.A.M.A.; Belal, A.; Abadir, M.F. Fabrication of geopolymer bricks using ceramic dust waste. Constr. Build. Mater. 2017, 157, 610–620. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Tahir, M.M.; Mirza, J. Properties of ceramic tile waste based alkali-activated mortars incorporating GBFS and fly ash. Constr. Build. Mater. 2019, 214, 355–368. [Google Scholar] [CrossRef]
- Keppert, M.; Vejmelková, E.; Bezdička, P.; Doleželová, M.; Čáchová, M.; Scheinherrová, L.; Pokorný, J.; Vyšvařil, M.; Rovnaníková, P.; Černý, R. Red-clay ceramic powders as geopolymer precursors: Consideration of amorphous portion and CaO content. Appl. Clay Sci. 2018, 161, 82–89. [Google Scholar] [CrossRef]
- Martirena, F.; Monzó, J. Vegetable ashes as Supplementary Cementitious Materials. Cem. Concr. Res. 2018, 114, 57–64. [Google Scholar] [CrossRef]
- Nalbantoglu, Z.; Gucbilmez, E. Improvement of calcareous expansive soils in semi-arid environments. J. Arid Environ. 2001, 47, 453–463. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Tortosa Masiá, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- EUR-Lex—32000D0532—ES. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000D0532:ES:HTML (accessed on 3 March 2020).
- Rosales, J.; Cabrera, M.; Beltrán, M.G.; López, M.; Agrela, F. Effects of treatments on biomass bottom ash applied to the manufacture of cement mortars. J. Clean. Prod. 2017, 154, 424–435. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Mañosa, J.; Maldonado-Alameda, A.; Quina, M.J.; Chimenos, J.M. Rapid sintering of weathered municipal solid waste incinerator bottom ash and rice husk for lightweight aggregate manufacturing and product properties. J. Clean. Prod. 2019, 232, 713–721. [Google Scholar] [CrossRef]
- Alam, Q.; Hendrix, Y.; Thijs, L.; Lazaro, A.; Schollbach, K.; Brouwers, H.J.H. Novel low temperature synthesis of sodium silicate and ordered mesoporous silica from incineration bottom ash. J. Clean. Prod. 2019, 211, 874–883. [Google Scholar] [CrossRef]
- James, K.A.; Thring, W.R.; Helle, S.; Ghuman, S.H. Ash Management Review: Applications of Biomass Bottom Ash. Energies 2012, 5, 3856. [Google Scholar] [CrossRef]
- UNE-EN 1097-7:2009 Tests for Mechanical and Physical Properties of Aggregates—Part 3: Determination of Loose Bulk Density and Voids. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0042553 (accessed on 16 September 2020).
- UNE-EN 772-16:2011 Methods of Test for Masonry Units—Part 16: Determination of Dimensions. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0047875 (accessed on 30 September 2020).
- UNE-EN 772-11:2011 Methods of Test for Masonry Units—Part 11: Determination of Water Absorption of Aggregate Concrete, Autoclaved Aerated Concrete, Manufactured Stone and Natural Stone Masonry Units due to Capillary Action and the Initial Rate of Water Absorption of Clay Masonry Units. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0047874 (accessed on 30 September 2020).
- UNE-EN 772-21:2011 Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0047877 (accessed on 30 September 2020).
- UNE-EN 772-7:1999 Methods of Test for Masonry Units—Part 7: Determination of Water Absorption of Clay Masonry Damp Proof Course Units by Boiling in Water. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0009121 (accessed on 30 September 2020).
- UNE-EN 772-4:1999 Methods of Test for Masonry Units—Part 4: Determination of Real and Bulk Density and of Total and Open Porosity for Natural Stone Masonry Units. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0009120 (accessed on 30 September 2020).
- UNE-EN 772-1:2011+A1:2016 Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?Tipo=N&c=N0056681 (accessed on 30 September 2020).
- UNE 67028:1997 EX Clay Bricks. Freezing Test. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0006752 (accessed on 30 September 2020).







| Samples Groups | Chamotte, % | BBA, % |
|---|---|---|
| 10C0A | 100 | 0 |
| 9C1A | 90 | 10 |
| 8C2A | 80 | 20 |
| 7C3A | 70 | 30 |
| 6C4A | 60 | 40 |
| 5C5A | 50 | 50 |
| 4C6A | 40 | 60 |
| 3C7A | 30 | 70 |
| 2C8A | 20 | 80 |
| 1C9A | 10 | 90 |
| 0C10A | 0 | 100 |
| Sample | Nitrogen, % | Carbon, % | Hydrogen, % | Sulfur, % |
|---|---|---|---|---|
| Chamotte | 0.00 ± 0.00 | 0.24 ± 0.01 | 0.08 ± 0.00 | 0.00 ± 0.00 |
| BBA | 0.05 ± 0.00 | 4.64 ± 0.14 | 0.48 ± 0.02 | 0.00 ± 0.00 |
| Sample | Loss on Ignition, % |
|---|---|
| Chamotte | 1.74 ± 0.10 |
| BBA | 8.16 ± 0.19 |
| Element | wt, % |
|---|---|
| Si | 27.32 ± 0.12 |
| Al | 8.16 ± 0.10 |
| Ca | 5.95 ± 0.10 |
| Fe | 4.57 ± 0.09 |
| K | 3.80 ± 0.09 |
| Mg | 1.92 ± 0.05 |
| Ti | 0.455 ± 0.023 |
| Sx | 0.119 ± 0.006 |
| Na | 0.201 ± 0.012 |
| P | 0.0965 ± 0.0048 |
| Mn | 0.0665 ± 0.0033 |
| Sr | 0.0523 ± 0.0030 |
| Zr | 0.0375 ± 0.0037 |
| V | 0.0209 ± 0.0018 |
| Ni | 0.0242 ± 0.0016 |
| Rb | 0.0208 ± 0.0043 |
| Cr | 0.0146 ± 0.0017 |
| Pt | 0.0162 ± 0.0039 |
| Cl | 0.0107 ± 0.0008 |
| Ru | 0.0070 ± 0.0026 |
| Total weight % oxygen | 45.39 ± 0.47 |
| Element | wt, % |
|---|---|
| K | 23.91 ± 0.19 |
| Si | 11.21 ± 0.10 |
| Ca | 11.10 ± 0.13 |
| Px | 3.58 ± 0.06 |
| Mg | 4.21 ± 0.08 |
| Al | 2.57 ± 0.06 |
| Fe | 1.33 ± 0.05 |
| Sx | 0.230 ± 0.011 |
| Na | 0.229 ± 0.019 |
| Cl | 0.255 ± 0.013 |
| Ti | 0.128 ± 0.006 |
| Sr | 0.0859 ± 0.0043 |
| Mn | 0.0442 ± 0.0022 |
| Cu | 0.0240 ± 0.0016 |
| Ni | 0.0221 ± 0.0012 |
| Cr | 0.0135 ± 0.0013 |
| Zr | 0.0106 ± 0.0027 |
| Rb | 0.0070 ± 0.0035 |
| Zn | 0.0047 ± 0.0016 |
| V | 0.0024 ± 0.0012 |
| Total weight % oxygen | 32.89 ± 0.36 |
| Groups | Chamotte, % | BBA, % | Red | Green | Blue |
|---|---|---|---|---|---|
| 10C0A | 100 | 0 | 379 ± 19 | 182 ± 9 | 115 ± 7 |
| 9C1A | 90 | 10 | 249 ± 12 | 119 ± 5 | 77 ± 3 |
| 8C2A | 80 | 20 | 253 ± 8 | 126 ± 7 | 84 ± 4 |
| 7C3A | 70 | 30 | 232 ± 10 | 126 ± 7 | 87 ± 4 |
| 6C4A | 60 | 40 | 192 ± 10 | 109 ± 5 | 78 ± 3 |
| 5C5A | 50 | 50 | 177 ± 10 | 107 ± 6 | 79 ± 3 |
| 4C6A | 40 | 60 | 170 ± 6 | 112 ± 4 | 85 ± 3 |
| 3C7A | 30 | 70 | 155 ± 8 | 115 ± 4 | 93 ± 3 |
| 2C8A | 20 | 80 | 147 ± 9 | 115 ± 6 | 97 ± 5 |
| 1C9A | 10 | 90 | 142 ± 8 | 122 ± 6 | 109 ± 5 |
| 0C10A | 0 | 100 | 118 ± 5 | 115 ± 6 | 110 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash. Materials 2021, 14, 199. https://doi.org/10.3390/ma14010199
Terrones-Saeta JM, Suárez-Macías J, Iglesias-Godino FJ, Corpas-Iglesias FA. Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash. Materials. 2021; 14(1):199. https://doi.org/10.3390/ma14010199
Chicago/Turabian StyleTerrones-Saeta, Juan María, Jorge Suárez-Macías, Francisco Javier Iglesias-Godino, and Francisco Antonio Corpas-Iglesias. 2021. "Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash" Materials 14, no. 1: 199. https://doi.org/10.3390/ma14010199
APA StyleTerrones-Saeta, J. M., Suárez-Macías, J., Iglesias-Godino, F. J., & Corpas-Iglesias, F. A. (2021). Development of Geopolymers as Substitutes for Traditional Ceramics for Bricks with Chamotte and Biomass Bottom Ash. Materials, 14(1), 199. https://doi.org/10.3390/ma14010199