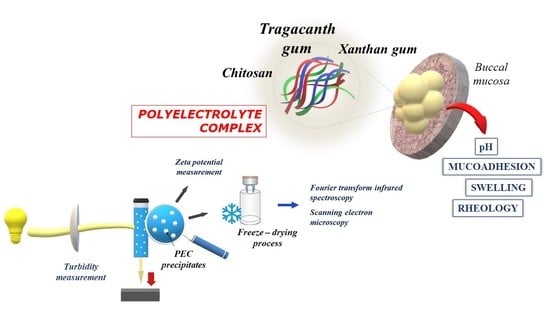
Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Hydrogels
2.3. pH Analysis
2.4. Evaluation of Mechanical Properties
2.5. Viscosity Measurement
2.6. Rheological Properties Analysis
2.7. Evaluation of Mucoadhesiveness by Gravimetric Test on an Inclined Temperature-Controlled Plane
2.8. Swelling Study
2.9. Turbidity Measurement
2.10. Zeta Potential and Conductivity Measurement
2.11. SEM Analysis
2.12. FTIR Analysis
3. Results and Discussion
3.1. Preparation and Physicochemical Characteristics of the Hydrogels
3.2. Mucoadhesion Properties
3.3. Swelling Ability
3.4. Turbidity
3.5. Zeta Potential and Conductivity
3.6. Morphology of the PECs
3.7. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A. Buccal bioadhesive drug delivery—A promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Seyfoori, A.; Amereh, M.; Gharaie, S.S.; Ghazali, H.S.; Ghazali, Z.S.; Khunjush, B.; Akbari, M. Stimuli-responsive hydrogels for local post-surgical drug delivery. Gels 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Shah, D.; Desai, T.R.; Noolvi, M.N. Mucoadhesive buccal films based on chitosan and carboxymethylated Feronia limonia fruit pulp mucilage interpolymer complex for delivery of opioid analgesics. Asian J. Pharm. 2016, 10, 137–143. [Google Scholar]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan—Based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Tejada, G.; Lamas, M.; Svetaz, L.; Salomón, C.; Alvarez, V.; Leonardi, D. Effect of drug incorporation technique and polymer combination on the performance of biopolymeric antifungal buccal films. Int. J. Pharm. 2018, 548, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, W.; Wu, Y.; He, Y.; Wu, T. Oxidative degradation of chitosan to the low molecular water-soluble chitosan over peroxotungstate as chemical scissors. PLoS ONE 2014, 9, e100743. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Bal, S.M.; Slütter, B.; Verheul, R.; Bouwstra, J.A.; Jiskoot, W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: Adjuvant- and site-dependent immunogenicity in mice. Eur. J. Pharm. Sci. 2012, 45, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K.; Szymańska, E. The effect of β-glycerophosphate crosslinking on chitosan cytotoxicity and physicochemical properties of hydrogels for vaginal application. Polymers 2015, 7, 2223–2244. [Google Scholar] [CrossRef] [Green Version]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS PharmSciTech 2017, 18, 936–946. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A.K.; Sinha, V.R. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech 2004, 5, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Palmeira-De-Oliveira, A.; Ribeiro, M.; Palmeira-De-Oliveira, R.; Gaspar, C.; Costa-De-Oliveira, S.; Correia, I.J.; Pina-Vaz, C.; Martinez-De-Oliveira, J.; Queiroz, J.A.; Rodrigues, A.G. Anti-candida activity of a chitosan hydrogel: Mechanism of action and cytotoxicity profile. Gynecol. Obstet. Investig. 2010, 70, 322–327. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Sonje, A.G.; Mahajan, H. Nasal inserts containing ondansetron hydrochloride based on Chitosan–gellan gum polyelectrolyte complex: In vitro–in vivo studies. Mater. Sci. Eng. C 2016, 64, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sakloetsakun, D.; Preechagoon, D.; Bernkop-Schnürch, A.; Pongjanyakul, T. Chitosan–gum arabic polyelectrolyte complex films: Physicochemical, mechanical and mucoadhesive properties. Pharm. Dev. Technol. 2016, 21, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, B.; Aldawsari, H.M.; Badr-Eldin, S.M. Optimized chitosan/anion polyelectrolyte complex based inserts for vaginal delivery of fluconazole: In vitro/in vivo evaluation. Pharmaceutics 2018, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Tay, F.R. Recent progress in the industrial and biomedical applications of tragacanth gum: A review. Carbohydr. Polym. 2019, 212, 450–467. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Le, X.T.; Turgeon, S.L. Rheological and structural study of electrostatic cross-linked xanthan gum hydrogels induced by β-lactoglobulin. Soft Matter 2013, 9, 3063–3073. [Google Scholar] [CrossRef]
- Kibbe, A.H. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: Washington, DC, USA, 2000. [Google Scholar]
- Zeng, N.; Dumortier, G.; Maury, M.; Mignet, N.; Boudy, V. Influence of additives on a thermosensitive hydrogel for buccal delivery of salbutamol: Relation between micellization, gelation, mechanic and release properties. Int. J. Pharm. 2014, 467, 70–83. [Google Scholar] [CrossRef]
- Menzel, C.; Jelkmann, M.; Laffleur, F.; Bernkop-Schnürch, A. Nasal drug delivery: Design of a novel mucoadhesive and in situ gelling polymer. Int. J. Pharm. 2017, 517, 196–202. [Google Scholar] [CrossRef]
- Pathak, K.; Sharma, V.; Akhtar, N.; Rastogi, P. Localization of fluconazole in oral cavity by preferential coating of buccoadhesive tablet for treatment of oral thrush. Int. J. Pharm. Investig. 2016, 6, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassem, A.; Ismail, F.A.; Naggar, V.F.; Aboulmagd, E. Preparation and evaluation of periodontal films based onpolyelectrolyte complexformation. Pharm. Dev. Technol. 2014, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdulhady, S.S.; Ibrahim, K.M.H. Preparation and evaluation of mebeverine hydrochloride as mucoadhesive buccal tablet for local anesthesia. Trop. J. Pharm. Res. 2017, 16, 1805–1812. [Google Scholar] [CrossRef] [Green Version]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination degree of deacetylation of chitosan: Comparison of methods. Prog. Chem. Appl. Chitin Deriv. 2012, 17, 5–20. [Google Scholar]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Mori, M.; Caramella, C.M. The role of chitosan as a mucoadhesive agent in mucosal drug delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 275–284. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Zerrouk, N.; Caramella, C.M. Mucoadhesive and penetration enhancement properties of three grades of hyaluronic acid using porcine buccal and vaginal tissue, Caco-2 cell lines, and rat jejunum. J. Pharm. Pharmacol. 2004, 56, 1083–1090. [Google Scholar] [CrossRef]
- Gilles, P.; Ghazali, F.A. Systemic oral mucosal drug delivery systems and delivery systems. In Oral Mucosal Drug Delivery; Rathbone, M.J., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2011; pp. 241–285. [Google Scholar]
- United States Pharmacopoeia and National Formulary (USP 41-NF 36); Pharmacopoeial Convention: Rockville, MD, USA, 2016.
- Yüksel, N.; Dinç, E.; Onur, F.; Baykara, T. Influence of swelling degree on release of nicardipine hydrochloride from acrylic microspheres prepared by solvent evaporation method. Pharm. Dev. Technol. 1998, 3, 115–121. [Google Scholar] [CrossRef]
- Strand, A.; Vähäsalo, L.; Ketola, A.; Salminen, K.; Retulainen, E.; Sundberg, A. In-situ analysis of polyelectrolyte complexes by flow cytometry. Cellulose 2018, 25, 3781–3795. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr. Polym. 2007, 67, 586–595. [Google Scholar] [CrossRef]
- Mohammadifar, M.A.; Musavi, S.M.; Kiumarsi, A.; Williams, P.A. Solution properties of targacanthin (water-soluble part of gum tragacanth exudate from Astragalus gossypinus). Int. J. Biol. Macromol. 2006, 38, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Brunchi, C.E.; Bercea, M.; Morariu, S.; Dascalu, M. Some properties of xanthan gum in aqueous solutions: Effect of temperature and pH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Nath, S.D.; Abueva, C.; Kim, B.; Lee, B.T. Chitosan–hyaluronic acid polyelectrolyte complex scaffold crosslinked with genipin for immobilization and controlled release of BMP-2. Carbohydr. Polym. 2015, 115, 160–169. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Silva, C. Rheological properties of aqueous dispersions of tragacanth gum and guar gum at different concentrations. J. Texture Stud. 2010, 41, 396–415. [Google Scholar] [CrossRef]
- Campos, J.C.; Ferreira, D.C.; Lima, S.; Lima, S.A.C.; Costa, P.J. Swellable polymeric particles for the local delivery of budesonide in oral mucositis. Int. J. Pharm. 2019, 566, 126–140. [Google Scholar] [CrossRef]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef] [Green Version]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Verhoeven, E.; Vervaet, C.; Remon, J.P. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2006, 63, 320–330. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Yarce, C.J.; Moreno, R.A.; Prieto, V.; Recalde, J. Natural gum-type biopolymers as potential modified nonpolar drug release systems. Carbohydr. Polym. 2018, 189, 31–38. [Google Scholar] [CrossRef]
- Spinks, G.M.; Lee, C.K.; Wallace, G.G.; Kim, S.I.; Kim, S.J. Swelling behavior of chitosan hydrogels in ionic liquid−water binary systems. Langmuir 2006, 22, 9375–9379. [Google Scholar] [CrossRef] [PubMed]
- Bobreshova, O.V.; Bobylkina, O.V.; Kulintsov, P.I.; Bobrinskaya, G.A.; Varlamov, V.P.; Nemtsev, S.V. Conductivity of aqueous solutions of low-molecular chitosan. Russ. J. Electrochem. 2004, 40, 694–697. [Google Scholar] [CrossRef]
- Cross, A.D. Introduction to Practical Infrared Spectroscopy; Butterworths: London, UK, 1960. [Google Scholar]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Sharma, V.; Devi, J. Site-specific tunable drug release from biocompatible tragacanth-cl-polyacrylamide polymer networks. Int. J. Plast. Technol. 2018, 22, 291–311. [Google Scholar] [CrossRef]
- Yuen, S.N.; Choi, S.M.; Phillips, D.L.; Ma, C.Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef] [PubMed]








| Formulation | Polymers Ratio (w/w) | |
|---|---|---|
| TG:CS 1 | XG:CS 1 | |
| F1 | 20:1 | – |
| F2 | 10:1 | – |
| F3 | 7:1 | – |
| F4 | 20:1 | 2:1 |
| F5 | 10:1 | 1:1 |
| F6 | 7:1 | 0.7:1 |
| Formulation | pH | Mechanical Properties | Viscosity (Pa·s) | ||
|---|---|---|---|---|---|
| Hardness (g) | Consistency (g·s) | Adhesiveness (g·s) | |||
| F1 | 5 | 191 ± 13 | 250 ± 6 | 250 ± 26 | 92 ± 0 |
| F2 | 5 | 24 ± 0 | 31 ± 0 | 27 ± 1 | 19 ± 0 |
| F3 | not determined 1 | ||||
| F4 | 5 | 210 ± 18 | 258 ± 11 | 272 ± 30 | 91 ± 0 |
| F5 | 5 | 107 ± 3 | 114 ± 1 | 100 ± 13 | 50 ± 2 |
| F6 | 5 | 34 ± 1 | 39 ± 1 | 40 ± 6 | 19 ± 1 |
| Polymers Ratio (w/w) | Zeta Potential (mV) [%] | Conductivity (mS/cm) | |
|---|---|---|---|
| TG:CS 1 | XG:CS 1 | ||
| 20:1 | – | Peak 1: −13.9 ± 5.7 [66.6 ± 14.1] Peak 2: 28.7 ± 5.8 [33.4 ± 14.1] | 0.48 ± 0.01 |
| 10:1 | – | 0.2 ± 0.3 2 | 0.72 ± 0.04 |
| 7:1 | – | 3.2 ± 0.1 2 | 0.97 ± 0.03 |
| 20:1 | 2:1 | –34.4 ± 12.4 2 | 0.50 ± 0.03 |
| 10:1 | 1:1 | –21.3 ± 2.6 2 | 0.82 ± 0.02 |
| 7:1 | 0.7:1 | 27.2 ± 3.3 2 | 0.27 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials 2021, 14, 86. https://doi.org/10.3390/ma14010086
Potaś J, Szymańska E, Basa A, Hafner A, Winnicka K. Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials. 2021; 14(1):86. https://doi.org/10.3390/ma14010086
Chicago/Turabian StylePotaś, Joanna, Emilia Szymańska, Anna Basa, Anita Hafner, and Katarzyna Winnicka. 2021. "Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application" Materials 14, no. 1: 86. https://doi.org/10.3390/ma14010086
APA StylePotaś, J., Szymańska, E., Basa, A., Hafner, A., & Winnicka, K. (2021). Tragacanth Gum/Chitosan Polyelectrolyte Complexes-Based Hydrogels Enriched with Xanthan Gum as Promising Materials for Buccal Application. Materials, 14(1), 86. https://doi.org/10.3390/ma14010086