Engineering Electronic Structure and Band Alignment of 2D Mg(OH)2 via Anion Doping for Photocatalytic Applications
Abstract
:1. Introduction
2. Methods
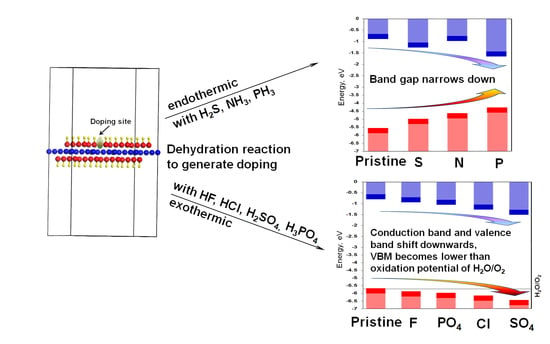
3. Results and Discussion
3.1. Electronic Structure of Bulk and 2D Mg(OH)2
3.2. Doping Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pilarska, A.A.; Klapiszewski, L.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg(OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, F.; Zhuang, Z.Y.; Lin, Z. A study of the potential application of nano-Mg(OH)2 in adsorbing low concentrations of uranyl tricarbonate from water. Nanoscale 2012, 4, 2423–2430. [Google Scholar] [CrossRef]
- Hao, J.W.; Dai, C.W.; Liu, Y.C.; Yang, Q. Removal of copper through adsorption by magnesium hydroxide nanorod. Desalin. Water Treat. 2017, 90, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.M.; Wang, F.; Lan, B.; Wang, D.C.; Liang, K.Z.; Li, T.Z.; Zhao, D.F.; Chen, J.J.; Lin, J.J.; Chan, W.; et al. Efficient treatment of anthraquinone dye wastewater by adsorption using sunflower torus-like magnesium hydroxide microspheres. Korean J. Chem. Eng. 2020, 37, 434–447. [Google Scholar] [CrossRef]
- Liu, W.P.; Xu, H.; Wang, Z.X.; Wang, X.M. Adsorption of water and fatty acids at magnesium hydroxide surface from an MDS perspective. Surf. Innov. 2019, 7, 304–316. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Fuchida, S.; Tokoro, C. Experimental study and surface complexation modeling of fluoride removal by magnesium hydroxide in adsorption and coprecipitation processes. J. Environ. Chem. Eng. 2020, 8, 10451. [Google Scholar] [CrossRef]
- Dong, C.X.; Cairney, J.; Sun, Q.H.; Maddan, O.L.; He, G.H.; Deng, Y.L. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J. Nanopart. Res. 2010, 12, 2101–2109. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R.; Csoka, L. Application of magnesium hydroxide nanocoatings on cellulose fibers with different refining degrees. RSC Adv. 2016, 6, 51583–51590. [Google Scholar] [CrossRef] [Green Version]
- Kaker, B.; Hribernik, S.; Mohan, T.; Kargl, R.; Kleinschek, K.S.; Pavlica, E.; Kreta, A.; Bratina, G.; Lue, S.J.; Bozic, M. Novel chitosan-Mg(OH)2-based nanocomposite membranes for direct alkaline ethanol fuel cells. ACS Sustain. Chem. Eng. 2019, 7, 19356–19368. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Z.; Zhang, Q.; Fan, L.Y.; Wang, R.; Yang, M.J.; Zhou, Y. 3D superhydrophobic sponge coated with magnesium hydroxide for effective oil/water mixture and emulsion separation. Ind. Eng. Chem. Res. 2020, 59, 11713–11722. [Google Scholar] [CrossRef]
- Ji, W.L.; Wang, H.R.; Yao, Y.J.; Wang, R.R. Mg(OH)2 and PDMS-coated cotton fabrics for excellent oil/water separation and flame retardancy. Cellulose 2019, 26, 6879–6890. [Google Scholar] [CrossRef]
- Ma, J.L.; Wang, X.; Li, J.; Chen, R.; Wei, J. Facile preparation of flame retardant cotton fabric via adhesion of Mg(OH)2 by the assistance of ionic liquid. Polymers 2020, 12, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Jiang, H.; Jing, B.N.; Zhang, M.; Liu, J.K.; Wang, S.H. Synthesis and characterisation of magnesium hydrate microspheres. Mater. Technol. 2014, 29, A28–A33. [Google Scholar] [CrossRef]
- Nabiyouni, G.; Ghanbari, D.; Karimzadeh, S.; Ghalehtaki, B.S. Sono-chemical synthesis Fe3O4-Mg(OH)2 nanocomposite and its photo-catalyst investigation in methyl orange degradation. J. Nanostruct. 2014, 4, 467–474. [Google Scholar]
- Flores-Flores, M.; Luevano-Hipolito, E.; Torres-Martinez, L.M.; Do, T.O. CO2 adsorption and photocatalytic reduction over Mg(OH)2/CuO/Cu2O under UV-visible light to solar fuels. Mater. Chem. Phys. 2019, 227, 90–97. [Google Scholar] [CrossRef]
- Luevano-Hipolito, E.; Martinez, L.M.T. Mg(OH)2 films prepared by ink-jet printing and their photocatalytic activity in CO2 reduction and H2O conversion. Top. Catal. 2018, 61, 1574–1584. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Vannoy, C.H.; Leblanc, R.M.; Wang, D.Z. Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram. Int. 2009, 35, 3355–3364. [Google Scholar] [CrossRef]
- Huang, C.H.; Jan, Y.L.; Lee, W.C. Investigation of Mg(O,OH) films prepared by chemical bath deposition as buffer layers for Cu(In,Ga)Se2 solar cells. J. Electrochem. Soc. 2011, 158, H879–H888. [Google Scholar] [CrossRef]
- Raza, S.M.; Ali, S.R.; Naeem, M.; Uddin, Z.; Qaseem, S.; Ali, S.I.; Shah, S.N. Tuning the bandgap in Co-doped Mg(OH)2 nanoparticles. Int. J. Mod. Phys. B 2019, 33, 1950182. [Google Scholar] [CrossRef]
- Murakami, T.; Honjo, T.; Kuji, T. DOS calculation analysis of new transparent conductor Mg(OH)2-C. Mater. Trans. 2011, 52, 1689–1692. [Google Scholar] [CrossRef]
- Miyazaki, H.; Mikami, R.; Yamada, A.; Konagai, M. Chemical-bath-deposited ZnO and Mg(OH)2 buffer layer for Cu(InGa)Se2 solar cells. Jpn. J. Appl. Phys. 2006, 45, 2618–2620. [Google Scholar] [CrossRef]
- Peiris, T.A.N.; Senthilarasu, S.; Wijayantha, K.G.U. Enhanced performance of flexible dye-sensitized solar cells: Electrodeposition of Mg(OH)2 on a nanocrystalline TiO2 electrode. J. Phys. Chem. C 2012, 116, 1211–1218. [Google Scholar] [CrossRef]
- Yum, J.H.; Nakade, S.; Kim, D.Y.; Yanagida, S. Improved performance in dye-sensitized solar cells employing TiO2 photoelectrodes coated with metal hydroxides. J. Phys. Chem. B 2006, 110, 3215–3219. [Google Scholar] [CrossRef] [PubMed]
- Keikhaei, M.; Ichimura, M. n-type and p-type semiconducting Cu-doped Mg(OH)2 thin films. Semicond. Sci. Technol. 2020, 35, 035020. [Google Scholar] [CrossRef]
- Keikhaei, M.; Ichimura, M. Fabrication of Mg(OH)2 thin films by electrochemical deposition with Cu catalyst. Thin Solid Films 2019, 681, 41–46. [Google Scholar] [CrossRef]
- Kang, J.; Keikhaei, M.; Li, T.; Ichimura, M. Galvanostatic electrochemical deposition of Cu-doped Mg(OH)2 thin films and fabrication of p-n homojunction. Mater. Res. Bull. 2021, 137, 111207. [Google Scholar] [CrossRef]
- Ichimura, M. Impurity doping in Mg(OH)2 for n-type and p-type conductivity control. Materials 2020, 13, 2972. [Google Scholar] [CrossRef]
- Honjo, T.; Chiba, M.; Kuji, T. Novel rare-elements free transparent conductor of Mg(OH)2-C compounds. J. Surf. Sci. Nanotechnol. 2009, 7, 791–794. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Yang, L.; Dai, B.; Geng, F.J.; Yang, Z.H.; Wang, P.; Gao, G.; Lei, P.; Han, J.C.; Ralchenko, V.; et al. Wide-range infrared transparency of hydrated magnesium-carbon films with high mobility for enhanced conductivity. Surf. Coat. Technol. 2019, 365, 70–75. [Google Scholar] [CrossRef]
- Suslu, A.; Wu, K.; Sahin, H.; Chen, B.; Yang, S.; Cai, H.; Aoki, T.; Horzum, S.; Kang, J.; Peeters, F.M.; et al. Unusual dimensionality effects and surface charge density in 2D Mg(OH)2. Sci. Rep. 2016, 6, 20525. [Google Scholar] [CrossRef] [Green Version]
- Yagmurcukardes, M.; Torun, E.; Senger, R.T.; Peeters, F.M.; Sahin, H. Mg(OH)2-WS2 van der Waals heterobilayer: Electric field tunable band-gap crossover. Phys. Rev. B 2016, 94, 195403. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.X.; Gao, Q.; Xiong, W.Q.; Du, J.; Zhao, X.; Wang, T.X.; Wei, Z.M.; Li, J.B. Electric field-tunable electronic structures of 2D alkaline-earth metal hydroxide-graphene heterostructures. J. Mater. Chem. C 2017, 5, 7230–7235. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Q.; Shen, J.; Yang, Y.; Qi, D.; Qi, Y.; Yuan, Q.; Zhong, C.; Sun, Z.; Sun, H. Computational screening of atomically thin two-dimensional nanomaterial-coated cs3sb heterostructures for high-performance photocathodes. J. Phys. Chem. C 2020, 124, 26396–26403. [Google Scholar] [CrossRef]
- Bacaksiz, C.; Dominguez, A.; Rubio, A.; Senger, R.T.; Sahin, H. h-AlN-Mg(OH)2 van derWaals bilayer heterostructure: Tuning the excitonic characteristics. Phys. Rev. B 2017, 95, 075423. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Mukhopadhyay, A.; Banerjee, L.; Sengupta, A.; Rahaman, H.; Sarkar, C.K. Ab initio study of mono-layer 2-D insulators (X-(OH)2 and h-BN) and their use in MTJ memory device. Microsyst. Technol. 2019, 25, 1909–1917. [Google Scholar] [CrossRef]
- Wang, B.J.; Li, X.H.; Cai, X.L.; Yu, W.Y.; Zhang, L.W.; Zhao, R.Q.; Ke, S.H. Blue Phosphorus/Mg(OH)2 van der Waals heterostructures as promising visible-light photocatalysts for water splitting. J. Phys. Chem. C 2018, 122, 7075–7080. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W.C. A two-dimensional vertical van der Waals heterostructure based on g-GaN and Mg(OH)2 used as a promising photocatalyst for water splitting: A first-principles calculation. J. Appl. Phys. 2019, 126, 065701. [Google Scholar] [CrossRef]
- Ren, K.; Yu, J.; Tang, W.C. First-principles study of two-dimensional van der Waals heterostructure based on ZnO and Mg(OH)2: A potential photocatalyst for water splitting. Phys. Lett. A 2019, 383, 125916. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.K.; Ren, K.; Chou, J.P.; Yu, J.; Sun, Z.M.; Sun, M.L. Transition-metal dichalcogenides/Mg(OH)2 van der Waals heterostructures as promising water-splitting photocatalysts: A first-principles study. PCCP 2019, 21, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Pishtshev, A.; Karazhanov, S.Z.; Klopov, M. Materials properties of magnesium and calcium hydroxides from first-principles calculations. Comput. Mater. Sci. 2014, 95, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Norskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Corà, F.; Alfredsson, M.; Mallia, G.; Middlemiss, D.S.; Mackrodt, W.C.; Dovesi, R.; Orlando, R. the performance of hybrid density functionals in solid state chemistry. In Principles and Applications of Density Functional Theory in Inorganic Chemistry II. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2004; Volume 113. [Google Scholar]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Catti, M.; Ferraris, G.; Hull, S.; Pavese, A. Static compression and H disorder in brucite, Mg(OH)2 to 11 GPa—A powder neutron-diffraction study. Phys. Chem. Min. 1995, 22, 200–206. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Zhang, P.X.; Song, S.H.; Yuan, Q.H.; Yang, P.; Ren, X.Z. Simulation of magnesium hydroxide surface and interface. J. Alloys Compd. 2014, 612, 315–322. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, S.; Wu, W.; Li, H.; Ren, T. Work function: A determining factor of the photodegradation rate of methyl orange via hollow octadecahedron Cu2O crystals. Phys. Chem. Chem. Phys. 2018, 20, 20117–20123. [Google Scholar] [CrossRef]
- Klein, A.; Körber, C.; Wachau, A.; Säuberlich, F.; Gassenbauer, Y.; Harvey, S.P.; Proffit, D.E.; Mason, T.O. Transparent conducting oxides for photovoltaics: Manipulation of fermi level, work function and energy band alignment. Materials 2010, 3, 4892–4914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.G.; Li, Y.L.; Sa, B.S.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Kovacic, Z.; Likozar, B.; Hus, M. Photocatalytic CO2 reduction: A review of ab initio mechanism, kinetics, and multiscale modeling simulations. ACS Catal. 2020, 10, 14984–15007. [Google Scholar] [CrossRef]
- Xu, S.M.; Pan, T.; Dou, Y.B.; Yan, H.; Zhang, S.T.; Ning, F.Y.; Shi, W.Y.; Wei, M. Theoretical and experimental study on (MMIII)-M-II-layered double hydroxides as efficient photocatalysts toward oxygen evolution from water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, X.; Marcel Veder, J.-P.; Shao, Z. Recent advances in anion-doped metal oxides for catalytic applications. J. Mater. Chem. A 2019, 7, 7280–7300. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhong, Y.; Zhou, W.; Shao, Z. Anion Doping: A new strategy for developing high-performance perovskite-type cathode materials of solid oxide fuel cells. Adv. Energy Mater. 2017, 7, 1700242. [Google Scholar] [CrossRef]
- Luo, H.; Takata, T.; Lee, Y.; Zhao, J.; Domen, K.; Yan, Y. Photocatalytic activity enhancing for titanium dioxide by co-doping with bromine and chlorine. Chem. Mater. 2004, 16, 846–849. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Second-generation photocatalytic materials: Anion-doped TiO2. J. Phys. Condens. Matter 2005, 18, 421–434. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Li, B.-Q.; Zhang, Q. A review of anion-regulated multi-anion transition metal compounds for oxygen evolution electrocatalysis. Inorg. Chem. Front. 2018, 5, 521–534. [Google Scholar] [CrossRef] [Green Version]





| Structure | Calculated | Experimental [51] | ||||
|---|---|---|---|---|---|---|
| a | b | c | a | b | c | |
| Bulk | 3.19 | 3.19 | 4.88 | 3.15 | 3.15 | 4.77 |
| 2D | 3.18 | 3.18 | - | |||
| Eg (PBE) (eV) | Eg (HSE06) (eV) | EWF (PBE) (eV) | EWF (HSE06) (eV) | |
|---|---|---|---|---|
| Bulk | 4.22 | 7.30 | 4.43 | 6.35 |
| 2D | 3.36 | 4.82 | 4.44 | 5.54 |
| Dopant | Ed (eV) | Bader Charge | Mg-Dopant Bond Length (Å) |
|---|---|---|---|
| F | −0.83 | +0.88 | 2.09 |
| Cl | −0.50 | +0.86 | 2.65 |
| S | 0.70 | +0.82 | 2.72 |
| N | 1.30 | +0.83 | 2.18 |
| P | 1.32 | +0.80 | 2.92 |
| SO4 | −1.14 | +0.92 | 2.40 |
| PO4 | −0.87 | +0.90 | 2.321 |
| Dopant | Eg (PBE) (eV) | Eg (HSE06) (eV) | EWF (PBE) (eV) | EWF (HSE06) (eV) |
|---|---|---|---|---|
| F | 3.38 | 4.89 | 4.50 | 5.19 |
| Cl | 3.36 | 4.82 | 4.96 | 5.66 |
| S | 2.76 | 3.86 | 4.29 | 4.60 |
| N | 2.48 | 3.79 | 3.69 | 4.24 |
| P | 1.83 | 2.69 | 3.32 | 3.85 |
| SO4 | 3.38 | 4.88 | 5.16 | 5.94 |
| PO4 | 3.36 | 4.87 | 4.757 | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Senevirathna, H.L.; Weerasinghe, P.V.T.; Wu, P. Engineering Electronic Structure and Band Alignment of 2D Mg(OH)2 via Anion Doping for Photocatalytic Applications. Materials 2021, 14, 2640. https://doi.org/10.3390/ma14102640
Wu S, Senevirathna HL, Weerasinghe PVT, Wu P. Engineering Electronic Structure and Band Alignment of 2D Mg(OH)2 via Anion Doping for Photocatalytic Applications. Materials. 2021; 14(10):2640. https://doi.org/10.3390/ma14102640
Chicago/Turabian StyleWu, Shunnian, Hasanthi L. Senevirathna, P. Vishakha T. Weerasinghe, and Ping Wu. 2021. "Engineering Electronic Structure and Band Alignment of 2D Mg(OH)2 via Anion Doping for Photocatalytic Applications" Materials 14, no. 10: 2640. https://doi.org/10.3390/ma14102640
APA StyleWu, S., Senevirathna, H. L., Weerasinghe, P. V. T., & Wu, P. (2021). Engineering Electronic Structure and Band Alignment of 2D Mg(OH)2 via Anion Doping for Photocatalytic Applications. Materials, 14(10), 2640. https://doi.org/10.3390/ma14102640