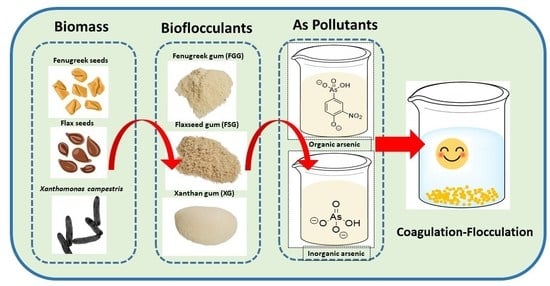
Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3. Thermogravimetric Analysis (TGA)
2.4. pH at Point-of-Zero-Charge (pHpzc)
2.5. Coagulation–Flocculation Process
2.6. Box–Behnken Experimental Design
2.7. Kinetic Studies
3. Results and Discussion
3.1. FT-IR Spectroscopy
3.2. Solids 13C-NMR Spectroscopy
3.3. Thermogravimetric Analysis (TGA)
3.4. pH at Point-of-Zero-Charge (pHpzc)
3.5. Box−Behnken Experimental Design
3.5.1. Main Effects of the Independent Variables on the Response Functions
3.5.2. Box−Behnken Analysis
3.5.3. Confirmation and Validation
3.6. Flocculation Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayarathne, H.N.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of Natural Organic Matter from Source Water: Review on Coagulants, Dual Coagulation, Alternative Coagulants, and Mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Ge, J.; Guha, B.; Lippincott, L.; Cach, S.; Wei, J.; Su, T.L.; Meng, X. Challenges of Arsenic Removal from Municipal Wastewater by Coagulation with Ferric Chloride and Alum. Sci. Total Environ. 2020, 725, 138351. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.; Campo, R.; Giustra, M.G.; De Marchis, M.; Di Bella, G. Bench Scale Continuous Coagulation-Flocculation of Saline Industrial Wastewater Contaminated by Hydrocarbons. J. Water Process Eng. 2020, 34, 101156. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/Flocculation in Dewatering of Sludge: A Review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S.A.; Ojha, N.; Das, N. Application of Aloe Vera Mucilage as Bioflocculant for the Treatment of Textile Wastewater: Process Optimization. Water Sci. Technol. 2020, 82, 2446–2459. [Google Scholar] [CrossRef]
- Kamel, R.; Afifi, S.M.; Kassem, I.A.A.; Elkasabgy, N.A.; Farag, M.A. Arabinoxylan and Rhamnogalacturonan Mucilage: Outgoing and Potential Trends of Pharmaceutical, Environmental, and Medicinal Merits. Int. J. Biol. Macromol. 2020, 165, 2550–2564. [Google Scholar] [CrossRef]
- Ajao, V.; Fokkink, R.; Leermakers, F.; Bruning, H.; Rijnaarts, H.; Temmink, H. Bioflocculants from Wastewater: Insights into Adsorption Affinity, Flocculation Mechanisms and Mixed Particle Flocculation Based on Biopolymer Size-Fractionation. J. Colloid Interface Sci. 2021, 581, 533–544. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Electrospinning of Double-Layer Chitosan-Flaxseed Mucilage Nanofibers for Sustained Release of Ziziphora Clinopodioides Essential Oil and Sesame Oil. Lwt 2021, 140, 110812. [Google Scholar] [CrossRef]
- Ding, H.H.; Qian, K.; Goff, H.D.; Wang, Q.; Cui, S.W. Structural and Conformational Characterization of Arabinoxylans from Flaxseed Mucilage. Food Chem. 2018, 254, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Bachmann, R.T. A Contemporary Review on Plant-Based Coagulants for Applications in Water Treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Kim, I.T.S.; Sethu, V.; Arumugasamy, S.K.; Selvarajoo, A. Fenugreek Seeds and Okra for the Treatment of Palm Oil Mill Effluent (POME)—Characterization Studies and Modeling with Backpropagation Feedforward Neural Network (BFNN). J. Water Process Eng. 2020, 37, 101500. [Google Scholar] [CrossRef]
- Mishra, S.; Kundu, K. Synthesis, Characterization and Applications of Polyacrylamide Grafted Fenugreek Gum (FG-g-PAM) as Flocculant: Microwave vs. Thermal Synthesis Approach. Int. J. Biol. Macromol. 2019, 141, 792–808. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Mo, C.; Zhao, K.; Chen, Z.; Wang, X. Influence of Fenugreek-Gum and Particle Size on Performance of Talc Flotation. Physicochem. Probl. Miner. Process. 2018, 54, 1026–1033. [Google Scholar] [CrossRef]
- Tangri, A. Trigonella Foenum-Graecum Mucilage: An Adsorbent for Removal of Sulphate Ions. Int. J. Adv. Res. Chem. Sci. 2014, 1, 34–42. [Google Scholar]
- Sousa, G.V.; Teles, V.L.G.; Pereira, E.G.; Modolo, L.V.; Costa, L.M. Interactions between As and Se upon Long Exposure Time and Effects on Nutrients Translocation in Golden Flaxseed Seedlings. J. Hazard. Mater. 2021, 402, 123565. [Google Scholar] [CrossRef]
- Cui, J.; Jing, C. A Review of Arsenic Interfacial Geochemistry in Groundwater and the Role of Organic Matter. Ecotoxicol. Environ. Saf. 2019, 183, 109550. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Sun, C.; Marhaba, T.F.; Zhang, W.; Zhang, Y. Arsenic Speciation by Sequential Extraction from As-Fe Precipitates Formed Under Different Coagulation Conditions. Water. Air. Soil Pollut. 2016, 227, 309. [Google Scholar] [CrossRef]
- Zang, S.; Zuo, Y.; Wang, J.; Liu, X.; Gomez, M.A.; Wei, L. Adsorption Removal of Roxarsone, Arsenite(III), and Arsenate(V) Using Iron-Modified Sorghum Straw Biochar and Its Kinetics. Acta Geochim. 2021, 40, 409–418. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. A Kinetic Uptake Study of Roxarsone Using Cross-Linked Chitosan Beads. Ind. Eng. Chem. Res. 2017, 56, 1704–1712. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A Review on Decontamination of Arsenic-Contained Water by Electrocoagulation: Reactor Configurations and Operating Cost along with Removal Mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Kong, D.; Wilson, L.D. Synthesis and Characterization of Cellulose-Goethite Composites and Their Adsorption Properties with Roxarsone. Carbohydr. Polym. 2017, 169, 282–294. [Google Scholar] [CrossRef]
- Kong, D.; Wilson, L.D. Uptake of Methylene Blue from Aqueous Solution by Pectin–Chitosan Binary Composites. J. Compos. Sci. 2020, 4, 95. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D.; Tabil, L.G. Biopolymer Flocculants and Oat Hull Biomass To Aid the Removal of Orthophosphate in Wastewater Treatment. Ind. Eng. Chem. Res. 2017, 56, 37–46. [Google Scholar] [CrossRef]
- Ibnul, N.K.; Tripp, C.P. A Solventless Method for Detecting Trace Level Phosphate and Arsenate in Water Using a Transparent Membrane and Visible Spectroscopy. Talanta 2021, 225, 122023. [Google Scholar] [CrossRef] [PubMed]
- Agbovi, H.K.; Wilson, L.D. Optimisation of Orthophosphate and Turbidity Removal Using an Amphoteric Chitosan-Based Flocculant–Ferric Chloride Coagulant System. Environ. Chem. 2019, 16, 599. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic Methyl Orange Removal from Aqueous Solutions by Activated Carbon Reinforced Conducting Polyaniline as Adsorbent: Synthesis, Characterization, Adsorption Behavior, Regeneration and Kinetics Study. J. Polym. Environ. 2022, 30, 886–895. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D. Kinetic Uptake Studies of Powdered Materials in Solution. Nanomaterials 2015, 5, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Reddy, C.K.; Huang, K.; Chen, L.; Xu, B. Hydrocolloidal Properties of Flaxseed Gum/Konjac Glucomannan Compound Gel. Int. J. Biol. Macromol. 2019, 133, 1156–1163. [Google Scholar] [CrossRef]
- Liang, S.; Li, X.; Ma, X.; Li, A.; Wang, Y.; Reaney, M.J.T.; Shim, Y.Y. A Flaxseed Heteropolysaccharide Stimulates Immune Responses and Inhibits Hepatitis B Virus. Int. J. Biol. Macromol. 2019, 136, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Minevich, I.E.; Osipova, L.L.; Nechiporenko, A.P.; Melnikova, M.I.; Tsyganova, T.B. IR-Spectroscopy of Polysaccharide Flaxseed (Linum usitatissimum L.) Products. Foods Raw Mater. 2019, 7, 274–282. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Mehmood, A.; Rashid, M.T.; Ali, N.; Naveed, M. Flaxseed Gum: Extraction, Bioactive Composition, Structural Characterization, and Its Potential Antioxidant Activity. J. Food Biochem. 2019, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; De Oliveira Petkowicz, C.L.; De Morais, S.A.L.; Terrones, M.G.H.; De Resende, M.M.; De Frana, F.P.; Cardoso, V.L. Characterization of Xanthan Gum Produced from Sugar Cane Broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Ćirić, A.; Medarević, Đ.; Čalija, B.; Dobričić, V.; Mitrić, M.; Djekic, L. Study of Chitosan/Xanthan Gum Polyelectrolyte Complexes Formation, Solid State and Influence on Ibuprofen Release Kinetics. Int. J. Biol. Macromol. 2020, 148, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Inphonlek, S.; Niamsiri, N.; Sunintaboon, P.; Sirisinha, C. Chitosan/Xanthan Gum Porous Scaffolds Incorporated with in-Situ-Formed Poly(Lactic Acid) Particles: Their Fabrication and Ability to Adsorb Anionic Compounds. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125263. [Google Scholar] [CrossRef]
- Qian, K.Y.; Cui, S.W.; Wu, Y.; Goff, H.D. Flaxseed Gum from Flaxseed Hulls: Extraction, Fractionation, and Characterization. Food Hydrocoll. 2012, 28, 275–283. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Carboxymethyl Derivatives of Flaxseed (Linum usitatissimum L.) Gum: Characterisation and Solution Rheology. Int. J. Food Sci. Technol. 2016, 51, 530–541. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Effect of Extraction Temperature on Composition, Structure and Functional Properties of Flaxseed Gum. Food Chem. 2017, 215, 333–340. [Google Scholar] [CrossRef]
- Kang, Y.; Li, P.; Zeng, X.; Chen, X.; Xie, Y.; Zeng, Y.; Zhang, Y.; Xie, T. Biosynthesis, Structure and Antioxidant Activities of Xanthan Gum from Xanthomonas Campestris with Additional Furfural. Carbohydr. Polym. 2019, 216, 369–375. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.Y.; Ahmad, A. Linum usitatissimum L. Seeds: Flax Gum Extraction, Physicochemical and Functional Characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave-Assisted Green Synthesis of Xanthan Gum Grafted Diethylamino Ethyl Methacrylate: An Efficient Adsorption of Hexavalent Chromium. Carbohydr. Polym. 2019, 222, 114989. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Fenugreek Seed Gum: Biological Properties, Chemical Modifications, and Structural Analysis—A Review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Physicochemical Properties and Aroma Profiles of Flaxseed Proteins Extracted from Whole Flaxseed and Flaxseed Meal. Food Hydrocoll. 2020, 104, 105731. [Google Scholar] [CrossRef]
- Hesami, F.; Bina, B.; Ebrahimi, A.; Amin, M. Arsenic Removal by Coagulation Using Ferric Chloride and Chitosan from Water. Int. J. Environ. Health Eng. 2013, 2, 17. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, Z.; Sun, B.; Sun, Y.; Wang, Q.; Guan, X. Arsenate and Arsenite Removal by FeCl3: Effects of PH, As/Fe Ratio, Initial As Concentration and Co-Existing Solutes. Sep. Purif. Technol. 2012, 92, 106–114. [Google Scholar] [CrossRef]
- Fox, D.I.; Stebbins, D.M.; Alcantar, N.A. Combining Ferric Salt and Cactus Mucilage for Arsenic Removal from Water. Environ. Sci. Technol. 2016, 50, 2507–2513. [Google Scholar] [CrossRef]
- Chiavola, A.; D’Amato, E.; Sirini, P.; Caretti, C.; Gori, R. Arsenic Removal from a Highly Contaminated Groundwater by a Combined Coagulation-Filtration-Adsorption Process. Water Air Soil Pollut. 2019, 230, 87. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H.; Pan, S.; Cheng, X.; Sun, Y.; Xu, Y. Effective and Simultaneous Removal of Organic/Inorganic Arsenic Using Polymer-Based Hydrated Iron Oxide Adsorbent: Capacity Evaluation and Mechanism. Sci. Total Environ. 2020, 742, 140508. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, T.; Zhang, H.; Liang, J.; Kong, W.; Yao, J.; Zhang, J.; Wang, J. Synthesis and Structure Characterization of Sulfated Galactomannan from Fenugreek Gum. Int. J. Biol. Macromol. 2019, 125, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-Based Flocculants: A Review of Recent Technologies. Environ. Sci. Pollut. Res. 2021, 28, 46934–46963. [Google Scholar] [CrossRef] [PubMed]
- Le, O.T.H.; Tran, L.N.; Doan, V.T.; Van Pham, Q.; Van Ngo, A.; Nguyen, H.H. Mucilage Extracted from Dragon Fruit Peel (Hylocereus Undatus) as Flocculant for Treatment of Dye Wastewater by Coagulation and Flocculation Process. Int. J. Polym. Sci. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Katubi, K.M.; Amari, A.; Harharah, H.N.; Eldirderi, M.M.; Tahoon, M.A.; Ben Rebah, F. Aloe Vera as Promising Material for Water Treatment: A Review. Processes 2021, 9, 782. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of Heavy Metals Present in Water from the Yautepec River Morelos México, Using Opuntia Ficus-Indica Mucilage. Environ. Adv. 2022, 7, 100160. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of PH and Contaminant Redox Form on the Competitive Removal of Arsenic and Antimony from Aqueous Media by Coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Lopez-Valdivieso, A.; Hernandez-Campos, D.J.; Peng, C.; Monroy-Fernandez, M.G.; Razo-Soto, I. Arsenic Removal from High-Arsenic Water by Enhanced Coagulation with Ferric Ions and Coarse Calcite. Water Res. 2006, 40, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.T.; Yamamoto, K.; Ahmed, M.F. A Low Cost Technique of Arsenic Removal from Drinking Water by Coagulation Using Ferric Chloride Salt and Alum. Water Supply 2002, 2, 281–288. [Google Scholar] [CrossRef]
- Asmel, N.K.; Yusoff, A.R.M.; Sivarama Krishna, L.; Majid, Z.A.; Salmiati, S. High Concentration Arsenic Removal from Aqueous Solution Using Nano-Iron Ion Enrich Material (NIIEM) Super Adsorbent. Chem. Eng. J. 2017, 317, 343–355. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Determination of Arsenic Removal Efficiency by Ferric Ions Using Response Surface Methodology. J. Hazard. Mater. 2009, 166, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Perez Mora, B.; Bellú, S.; Mangiameli, M.F.; Frascaroli, M.I.; González, J.C. Response Surface Methodology and Optimization of Arsenic Continuous Sorption Process from Contaminated Water Using Chitosan. J. Water Process Eng. 2019, 32, 100913. [Google Scholar] [CrossRef]





| Independent Factors | Units | Symbol | Coded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| FeCl3 | mg L−1 | A | 25 | 37.5 | 50 |
| Gum | mg L−1 | B | 1 | 60.5 | 120 |
| Settling time | min | C | 10 | 50 | 90 |
| Coefficients | S1 | S2 | ||||
|---|---|---|---|---|---|---|
| FSG | FGG | XG | FSG | FGG | XG | |
| Const | 88.0 | 87.0 | 88.5 | 76.167 | 67.5 | 66.667 |
| FeCl3 (A) | −10.25 | −30.938 | −18.25 | −4.3125 | −10.313 | −9.1875 |
| GUM (B) | 2.125 | −1.3125 | −2.6875 | −0.0625 | 3.25 | −12.375 |
| ST (C) | 6.875 | 3.0 | 5.9375 | 6.0 | 1.8125 | 2.3125 |
| AB | 7.375 | 1.25 | 13.0 | −7.0 | 4.625 | 4.0 |
| AC | −4.375 | −1.375 | −1.0 | −2.125 | 0.5 | −0.125 |
| BC | −1.375 | 1.125 | 3.375 | −5.625 | −0.625 | 2.75 |
| AA | −28.938 | −47.875 | −34.563 | −16.708 | −12.625 | −17.396 |
| BB | −4.9375 | −4.375 | −18.188 | −19.958 | −27.5 | −17.021 |
| CC | −4.9375 | −7.5 | −6.4375 | −15.583 | −24.875 | −6.8958 |
| GUM | GUM Dose (mg L−1) | FeCl3 (mg L−1) | Settling Time (min) | As Removal (%) (50 mg L−1) | |
|---|---|---|---|---|---|
| S1 (Roxarsone) | |||||
| Predicted | Experimental | ||||
| FSG | 64 | 35 | 81 | 91.8 | 92.0 ± 0.6 |
| FGG | 52 | 33 | 58 | 92.5 | 92.3 ± 0.1 |
| XG | 50 | 34 | 68 | 92.6 | 92.8 ± 0.1 |
| S2 (Arsenate) | |||||
| Predicted | Experimental | ||||
| FSG | 60 | 36 | 58 | 77.0 | 77.0 ± 0.1 |
| FGG | 62 | 32 | 51 | 69.4 | 69.6 ± 0.6 |
| XG | 37 | 34 | 54 | 70.6 | 70.6 ± 0.9 |
| Flocculant | Concentration (mg L−1) | Coagulant | Concentration (mg L−1) | As (V) (mg L−1) | pH | RE (%) | Reference |
|---|---|---|---|---|---|---|---|
| Aloe vera gum | 2 | PAC | 3 | 0.2–1 | 5 | 92.6 | [53] |
| Chitosan | 0.5 | FeCl3 | 0.2–2 | 7 | ~100 | [45] | |
| Opuntia ficus- indica gum | 350 | - | - | 0.002–0.01 | 5.9 | 70 | [54] |
| - | - | FeCl3 | 27.029 | 1 | 5 | 98 | [55] |
| - | - | Fe2(SO4)3 | 100 | 5 | 6 | 99 | [56] |
| - | - | Al2(SO4)3 | 25–50 | 0.065–0.216 | 7–8 | 81 | [57] |
| Flaxseed gum | 64 | FeCl3 | 35 | 50 | 7 | 90 | This work |
| Fenugreek gum | 52 | FeCl3 | 33 | 50 | 7 | 90 | This work |
| Xanthan gum | 50 | FeCl3 | 34 | 50 | 7 | 93 | This work |
| Flaxseed gum | 60 | FeCl3 | 36 | 50 | 7.5 | 77 | This work |
| Fenugreek gum | 62 | FeCl3 | 32 | 50 | 7.5 | 69.6 | This work |
| Xanthan gum | 37 | FeCl3 | 34 | 50 | 7.5 | 70.6 | This work |
| GUM | Pseudo-First-Order (PFO) Model | Pseudo-Second-Order (PSO) Model | ||||
|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | |
| (min−1) | (mg g−1) | (min−1) | (mg g−1) | |||
| S1 (Roxarsone) | ||||||
| FSG | 0.168 | 518.6 ± 10.8 | 0.952 | 3.6 × 10−4 | 580.3 ± 23.6 | 0.897 |
| FGG | 0.147 | 607.5 ± 16.7 | 0.924 | 2.7 × 10−4 | 682.3 ± 32.9 | 0.865 |
| XG | 0.163 | 602.6 ± 11.5 | 0.956 | 3.2 × 10−4 | 669.5 ± 25.1 | 0.901 |
| S2 (Arsenate) | ||||||
| FSG | 0.138 | 398.9 ± 13.7 | 0.896 | 3.7 × 10−4 | 451.2 ± 25.6 | 0.842 |
| FGG | 0.112 | 296.7 ± 9.8 | 0.915 | 3.7 × 10−4 | 343.2 ± 19.6 | 0.869 |
| XG | 0.168 | 537.5 ± 8.6 | 0.967 | 3.9 × 10−4 | 595.1 ± 16.8 | 0.937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venegas-García, D.J.; Wilson, L.D. Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water. Materials 2022, 15, 8691. https://doi.org/10.3390/ma15238691
Venegas-García DJ, Wilson LD. Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water. Materials. 2022; 15(23):8691. https://doi.org/10.3390/ma15238691
Chicago/Turabian StyleVenegas-García, Deysi J., and Lee D. Wilson. 2022. "Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water" Materials 15, no. 23: 8691. https://doi.org/10.3390/ma15238691
APA StyleVenegas-García, D. J., & Wilson, L. D. (2022). Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water. Materials, 15(23), 8691. https://doi.org/10.3390/ma15238691