Long-Term Leaching Behavior of Organic and Inorganic Pollutants after Wet Processing of Solid Waste Materials
Abstract
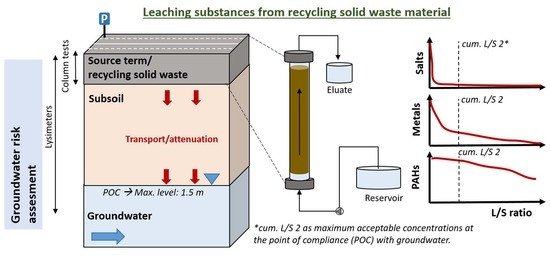
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Analytics
2.3.1. Turbidity, Electrical Conductivity, Ion Chromatography and DOC
2.3.2. Metals, Phenols, EOX, PCBs, PHCs, Cyanide and Herbicides
2.3.3. Polycyclic Aromatic Hydrocarbons (PAHs)
3. Results and Discussion
3.1. Pollutant Screening in Solid Fractions
3.2. Contaminant Concentrations in Eluates of Short Leaching Tests and Washing Water
3.3. Comparison between Short and Extensive Column Tests: The Importance of Compliance Testing
3.4. Typical Release Patterns of Groups of Substances and Fitting of the Advection-Dispersion Transport Model
3.5. Dynamics of Contaminant Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Concentration Measurements
| Samples | Sb | As | Ba | Pb | B | Cd | Cr | Co | Cu | Mo | Hg | Ni | Se | Tl | V | Zn | PHC a | PCBs b | BaP | 16PAHs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/kg] | [mg/kg] | [mg/kg] | [mg/kg] | |||||||||||||||||
| RB-In | 3.3 | 12.9 | 135 | 31 | 68.5 | 0.64 | 44.8 | 15.2 | 81.4 | 2.07 | 0.12 | 62.9 | 1.71 | 0.21 | 39.5 | 333 | <25 | 0.003 | 0.92 | 12.50 |
| RB-U | 3.8 | 16 | 163 | 44.1 | 81.7 | 0.65 | 47.5 | 16 | 107 | 2.07 | 0.21 | 63.7 | 1.6 | 0.29 | 40.5 | 523 | 63 | 0.005 | 1.62 | 21.83 |
| RB-S | 2.9 | 9.3 | 139 | 16.8 | 49.5 | 0.35 | 38.8 | 13.9 | 48.5 | 1.6 | 0.06 | 67.8 | 1.76 | 0.1 | 36.4 | 125 | 86 | 0.009 | 0.26 | 4.08 |
| CDW1-In | 0.23 | 7.2 | 39.6 | 9.91 | 28 | 0.23 | 15.1 | 4.06 | 8.1 | 0.18 | <0.05 | 15.9 | 2.09 | 0.11 | 21.5 | 34.9 | <25 | <0.002 | 0.65 | 7.91 |
| CDW1-U | 0.50 | 14.9 | 113 | 27.7 | 66.2 | 0.37 | 32.5 | 11.8 | 21.3 | 0.34 | 0.075 | 43.6 | 2.07 | 0.29 | 43.8 | 85.7 | 98 | 0.016 | 0.93 | 10.68 |
| CDW1-S | 0.20 | 7.9 | 36.9 | 13.8 | 22.6 | 0.12 | 13.6 | 3.82 | 6.6 | 0.35 | 0.05 | 16.2 | <0.2 | <0.1 | 17.2 | 32.6 | 139 | 0.004 | 0.27 | 3.717 |
| CDW1-G | 0.19 | 5.4 | 19.5 | 3.86 | 19.5 | 0.25 | 9.03 | 2.24 | 3.6 | 0.21 | <0.05 | 9.5 | 1.84 | <0.1 | 11.7 | 20.6 | <25 | <0.002 | 0.0 | 0.005 |
| CDW2-In | 0.44 | 5.4 | 90.5 | 15.8 | 32.6 | 0.16 | 15.2 | 4.79 | 17.3 | 0.33 | 0.07 | 16.5 | 0.31 | 0.15 | 19.8 | 45.4 | 121 | 0.011 | 6.03 | 81.89 |
| CDW2-U | 1.3 | 12.6 | 211 | 49.2 | 80.1 | 0.42 | 10.6 | 10.3 | 44.1 | 1.2 | 0.21 | 39 | 0.69 | 0.39 | 36.6 | 131 | 191 | 0.041 | 3.96 | 43.31 |
| CDW2-S | 0.61 | 4.6 | 66.1 | 15.6 | 22.8 | 0.25 | 13.8 | 4.31 | 32.7 | 0.50 | <0.05 | 15.2 | 0.23 | 0.1 | 15.3 | 55.8 | 85 | 0.016 | 2.73 | 38.94 |
| CDW2-G | 0.65 | 4.3 | 53.4 | 13 | 18.1 | 0.17 | 9.2 | 2.4 | 7.4 | 0.21 | <0.05 | 9.6 | 0.2 | 0.1 | 11.7 | 17 | 104 | <0.002 | 0.08 | 0.94 |
| CDW3-In | 0.65 | 6.7 | 62 | 26.6 | 38.1 | 0.43 | 17.4 | 6.22 | 17.2 | 0.17 | 0.21 | 22 | 0.37 | 0.14 | 20.8 | 68 | 54 | <0.002 | 0.33 | 4.18 |
| CDW3-U | 1.2 | 14.2 | 148 | 45.6 | 71 | 0.51 | 28.9 | 11.2 | 35.3 | 0.65 | 0.31 | 41 | 0.81 | 0.37 | 33.9 | 120 | 123 | 0.012 | 0.90 | 17.33 |
| CDW3-S | 0.42 | 9.2 | 74 | 26.1 | 27.2 | 0.36 | 13.8 | 5.13 | 13.9 | 0.32 | 0.05 | 17.4 | 0.45 | 0.11 | 18.7 | 35.9 | 93 | <0.002 | 0.36 | 6.56 |
| CDW3-G | 0.25 | 6.0 | 38.5 | 7.37 | 24.3 | 0.20 | 10.3 | 3.6 | 8.4 | 0.19 | <0.05 | 14.1 | 0.38 | 0.1 | 15.4 | 27.5 | 79 | <0.002 | 0.51 | 6.62 |
| Threshold c | - | 10 | - | 40 | - | 0.4 | 30 | - | 20 | - | 0.2 | 15 | - | 0.5 | - | 60 | 300 d | 0.05 | 0.3 | 3 |
| Samples | Atrazine | Simazine | Bromacil | Desethyl-atrazine | Diuron | Glyphosate | Ampa |
|---|---|---|---|---|---|---|---|
| [µg/L] | [µg/L] | [µg/L] | [µg/L] | [µg/L] | [µg/L] | [µg/L] | |
| RB-In | - | - | - | - | - | - | 0.29 |
| RB-U | 0.05 | 0.04 | - | 0.045 | 0.046 | - | - |
| RB-S | 0.093 | 0.19 | 0.026 | 0.074 | 0.076 | 0.31 | 1.5 |
| RB-G | 0.027 | 0.042 | - | - | 0.032 | 0.15 | 0.43 |
| RB-WW | 0.062 | 0.1 | 0.029 | 0.079 | 0.023 | - | 0.29 |
| GFS value a | 0.1 | 0.1 | 0.1 | 0.1 | 0.05 | 0.1 | 0.1 |
| Threshold b | 0.2 | 0.2 | 0.2 | - | 0.1 | 0.2 | 2.5 |
Appendix B. Long-Term Leaching Behavior Described by the Advection-Dispersion Equation
References
- BAU. Mineralische Bauabfälle Monitoring 2018. In Bericht zum Aufkommen und zum Verbleib Mineralischer Bauabfälle im Jahr 2018; Kreislaufwirtschaft BAU: Berlin, Germany, 2021. [Google Scholar]
- Allegrini, E.; Butera, S.; Kosson, D.S.; Van Zomeren, A.; Van der Sloot, H.A.; Astrup, T.F. Life cycle assessment and residue leaching: The importance of parameter, scenario and leaching data selection. Waste Manag. 2015, 38, 474–485. [Google Scholar] [CrossRef]
- Grathwohl, P.; Susset, B. Comparison of percolation to batch and sequential leaching tests: Theory and data. Waste Manag. 2009, 29, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Finkel, M.; Grathwohl, P. Impact of pre-equilibration and diffusion limited release kinetics on effluent concentration in column leaching tests: Insights from numerical simulations. Waste Manag. 2017, 63, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Vollpracht, A.; Markus, P.; Linnemann, V. Optimization of a German short term percolation test to determine the leaching of granular materials. Waste Manag. 2020, 105, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, U.; Bandow, N.; Bredow, A.; Mathies, H.; Piechotta, C. Column leaching tests on soils containing less investigated organic pollutants. J. Geochem. Explor. 2014, 147, 291–297. [Google Scholar] [CrossRef]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. Percolation and batch leaching tests to assess release of inorganic pollutants from municipal solid waste incinerator residues. Waste Manag. 2011, 31, 236–245. [Google Scholar] [CrossRef]
- Naka, A.; Yasutaka, T.; Sakanakura, H.; Kalbe, U.; Watanabe, Y.; Inoba, S.; Takeo, M.; Inui, T.; Katsumi, T.; Fujikawa, T.; et al. Column percolation test for contaminated soils: Key factors for standardization. J. Hazard. Mater. 2016, 320, 326–340. [Google Scholar] [CrossRef] [Green Version]
- López Meza, S.; Kalbe, U.; Berger, W.; Simon, F.-G. Effect of contact time on the release of contaminants from granular waste materials during column leaching experiments. Waste Manag. 2010, 30, 565–571. [Google Scholar] [CrossRef]
- DIN 19528. Elution von Feststoffen–Perkolationsverfahren zur Gemeinsamen Untersuchung des Elutionsverhaltens von Anorganischen und Organischen Stoffen; Ausgabe 01/2009; Beuth: Berlin, Germany, 2009. [Google Scholar]
- Susset, B.; Grathwohl, P. Leaching standards for mineral recycling materials—A harmonized regulatory concept for the upcoming German Recycling Decree. Waste Manag. 2011, 31, 201–214. [Google Scholar] [CrossRef]
- Liu, B.; Finkel, M.; Grathwohl, P. Mass transfer principles in column percolation tests: Initial conditions and tailing in heterogeneous materials. Materials 2021, 14, 4708. [Google Scholar] [CrossRef]
- Grathwohl, P. On equilibration of pore water in column leaching tests. Waste Manag. 2014, 34, 908–918. [Google Scholar] [CrossRef]
- Dabo, D.; Badreddine, R.; De Windt, L.; Drouadaine, I. Ten-year chemical evolution of leachate and municipal solid waste incineration bottom ash used in a test road site. J. Hazard. Mater. 2009, 172, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bordado, J.C.M.; Quinta-Ferreira, R.M. The influence of pH on the leaching behaviour of inorganic components from municipal solid waste APC residues. Waste Manag. 2009, 29, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Van Zomeren, A.; Comans, R.N.J. Contribution of natural organic matter to copper leaching from municipal solid waste incinerator bottom ash. Environ. Sci. Technol. 2004, 38, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.C.M.A.; Lourençlo, J.C.N. The influence of organic matter content of contaminated soils on the leaching rate of heavy metals. Environ. Prog. 2000, 19, 53–58. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Kosson, D.S.; Brown, K.G.; Fagnant, D.P.; Helms, G.; Thorneloe, S.A. Methodology for scenario-based assessments and demonstration of treatment effectiveness using the Leaching Environmental Assessment Framework (LEAF). J. Hazard. Mater. 2021, 406, 124635. [Google Scholar] [CrossRef]
- BGBI. Verordnung zur Einführung Einer Ersatzbaustoffverordnung, zur Neufassung der Bundes-Bodenschutz- und Altlastenverordnung und zur Änderung der Deponieverordnung und der Gewerbeabfallverordnung; Bundesrat: Köln, Germany, 2021; p. 155.
- LAWA. Derivation of insignificant threshold values for groundwater. In Länderarbeitsgemeinschaft Wasser; Ministerium für Umwelt, Klima und Energiewirtschaft: Stuttgart, Germany, 2017. [Google Scholar]
- Kalbe, U.; Simon, F.-G. Potential use of incineration bottom ash in construction: Evaluation of the environmental impact. Waste Biomass Valor 2020, 11, 7055–7065. [Google Scholar] [CrossRef]
- Zand, A.D.; Grathwohl, P.; Nabibidhendi, G.; Mehrdadi, N. Determination of leaching behaviour of polycyclic aromatic hydrocarbons from contaminated soil by column leaching test. Waste Manag. Res. J. Sustain. Circ. Econ. 2010, 28, 913–920. [Google Scholar] [CrossRef]
- Quenea, K.; Lamy, I.; Winterton, P.; Bermond, A.; Dumat, C. Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 2009, 149, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Diotti, A.; Perèz Galvin, A.; Piccinali, A.; Plizzari, G.; Sorlini, S. Chemical and Leaching Behavior of Construction and Demolition Wastes and Recycled Aggregates. Sustainability 2020, 12, 10326. [Google Scholar] [CrossRef]
- Butera, S.; Hyks, J.; Christensen, T.H.; Astrup, T.F. Construction and demolition waste: Comparison of standard up-flow column and down-flow lysimeter leaching tests. Waste Manag. 2015, 43, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; van der Sloot, H.A.; Comans, R.N.J. The leaching of major and trace elements from MSWI bottom ash as a function of pH and time. Appl. Geochem. 2006, 21, 335–351. [Google Scholar] [CrossRef]
- Schwientek, M.; Rügner, H.; Beckingham, B.; Kuch, B.; Grathwohl, P. Integrated monitoring of particle associated transport of PAHs in contrasting catchments. Environ. Pollut. 2013, 172, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, L.; Celi, L.; Cignetti, A.; Marsan, F.A. Influence of soil organic matter on the leaching of polycyclic aromatic hydrocarbons in soil. J. Environ. Sci. Health Part B 2002, 37, 187–199. [Google Scholar] [CrossRef]
- Totsche, K.U.; Jann, S.; Kögel-Knabner, I. Release of Polycyclic Aromatic hydrocarbons, dissolved organic carbon, and suspended matter from disturbed NAPL-contaminated gravelly soil material. Vadose Zone J. 2006, 5, 469–479. [Google Scholar] [CrossRef]
- Rügner, H.; Schwientek, M.; Milačič, R.; Zuliani, T.; Vidmar, J.; Paunović, M.; Laschou, S.; Kalogianni, E.; Skoulikidis, N.T.; Diamantini, E.; et al. Particle bound pollutants in rivers: Results from suspended sediment sampling in Globaqua River Basins. Sci. Total Environ. 2019, 647, 645–652. [Google Scholar] [CrossRef]
- Ogata, A.; Banks, R.B. A Solution of the Differential Equation of Longitudinal Dispersion in Porous Media. In Fluid Movement in Earth Materials; U.S. Geological Survey: Washington, DC, USA, 1961. [Google Scholar]








| Parameter | RB | CDW1 | CDW2 | CDW3 | CDW3 Extensive Tests a | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In d | U e | S | G | In | U | S | G | In | U | S | G | In | U | S | G | In | U | S | |
| Water content [%] b | 3.13 | 31.3 | 10.6 | 1.8 | 8.6 | 38.8 | 11.9 | 2.51 | 10.3 | 36.11 | 8.08 | 1.97 | 7.43 | 35.37 | 12.06 | 4.57 | 15 | 22 | 12.06 |
| Dry sample [g] | 1615 | 251 | 664 | 914 | 2417 | 182 | 676 | 882 | 1454 | 199 | 784 | 841 | 1326 | 193 | 873 | 840 | 927 | 172 | 839 |
| Quartz sand [g] | 1987 | 2225 | - | - | 2656 | 2208 | - | - | 2052 | 2192 | - | - | 2040 | 2208 | - | - | 1632 | 2507 | - |
| Filling volume [cm3] b | 1193 | 1078 | 550 | 560 | 1116 | 1155 | 530 | 550 | 553 | 1116 | 550 | 530 | 550 | 1193 | 550 | 540 | 1155 | 1116 | 550 |
| Porosity [%] b | 51 | 48 | 56 | 41 | 30 | 40 | 52 | 41 | 31 | 38 | 46 | 41 | 34 | 40 | 41 | 42 | 34 | 41 | 44 |
| Flow velocity [mL/min] | 1.96 | 1.03 | 0.79 | 0.85 | 1.11 | 1.52 | 0.84 | 0.41 | 0.54 | 1.5 | 0.83 | 0.74 | 0.62 | 1.55 | 0.74 | 0.72 | 1.30 | 1.48 | 0.80 |
| TOC [mg C/g] c | 25.1 | 11.5 | 0.60 | - | 9.61 | 5.14 | 1.42 | - | 13.4 | 5.59 | 2.62 | - | 13.7 | 4.42 | 1.00 | - | (see CDW3) | ||
| Threshold and Material Values | F− | Cl− | SO42− | As | Cr | Cu | Mo | Ni | Se | V | BaP | PAHs f | PCBs g | PHC h | Phenol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | mg/L | mg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| GFS values a | 900 | 250 | 250 | 3.2 | 3.4 | 5.4 | 35 | 7 | 3 | 4 | 0.01 | 0.2 | 0.01 | 100 | 8 |
| BM-F0* b | - | - | 250 | 12 | 15 | 30 | 55 | 30 | - | 30 | - | 0.3 | 0.02 | 150 | 12 |
| BM-F1 c | - | - | 450 | 20 | 150 | 110 | 55 | 20 | - | 55 | - | 1.5 | 0.02 | 160 | 60 |
| BM-F2 d | - | - | 450 | 85 | 290 | 170 | 55 | 20 | - | 450 | - | 3.8 | 0.02 | 160 | 60 |
| RC-1 e | - | - | 600 | - | 150 | 110 | - | - | - | 120 | - | 4 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Espinoza, M.; Susset, B.; Grathwohl, P. Long-Term Leaching Behavior of Organic and Inorganic Pollutants after Wet Processing of Solid Waste Materials. Materials 2022, 15, 858. https://doi.org/10.3390/ma15030858
Prieto-Espinoza M, Susset B, Grathwohl P. Long-Term Leaching Behavior of Organic and Inorganic Pollutants after Wet Processing of Solid Waste Materials. Materials. 2022; 15(3):858. https://doi.org/10.3390/ma15030858
Chicago/Turabian StylePrieto-Espinoza, Maria, Bernd Susset, and Peter Grathwohl. 2022. "Long-Term Leaching Behavior of Organic and Inorganic Pollutants after Wet Processing of Solid Waste Materials" Materials 15, no. 3: 858. https://doi.org/10.3390/ma15030858
APA StylePrieto-Espinoza, M., Susset, B., & Grathwohl, P. (2022). Long-Term Leaching Behavior of Organic and Inorganic Pollutants after Wet Processing of Solid Waste Materials. Materials, 15(3), 858. https://doi.org/10.3390/ma15030858