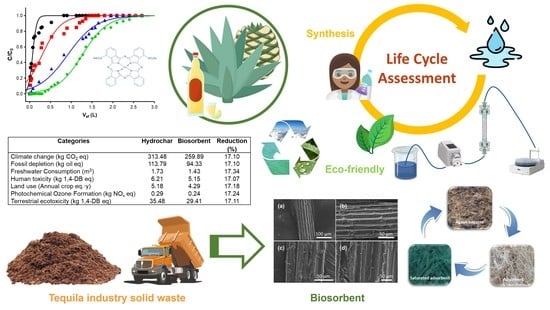
Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Adsorbent Materials
2.2. Adsorption Studies
2.3. Life Cycle Assessment (LCA) Study
3. Results and Discussion
3.1. Characterization of the Adsorbent Materials
3.2. Adsorption Studies of DB-86 onto Carbon Materials
3.3. Life Cycle Assessment of the Adsorbent Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federación, D.O. de la NOM-006-SCFI-2012, Norma Oficial Mexicana: Bebidas Alcohólicas Tequila Especificaciones; Dirección General de Normas: México City, México, 2012; pp. 1–21. [Google Scholar]
- Iñiguez, G.; Acosta, N.; Martínez, L.; Parra, J.; González, O. Utilización de Subproductos de La Industria Tequilera. Parte 7. Compostaje de Bagazo de Agave y Vinazas Tequileras. Rev. Int. Contam. Ambient 2005, 21, 37–50. [Google Scholar]
- Warren-vega, W.M.; Aguilar-hernández, D.E.; Zárate-guzmán, A.I.; Romero-cano, L.A.; Campos-rodríguez, A. Development of a Predictive Model for Agave Prices Employing Environmental, Economic, and Social Factors: Towards a Planned Supply Chain for Agave–Tequila Industry. Foods 2022, 11, 1138. [Google Scholar] [CrossRef]
- López-López, A.; Contreras-Ramos, S.M. Tratamiento de Efluentes y Aprovechamiento de Residuos. In Ciencia y Tecnología del Tequila: Avances y Perspectivas; CIATEJ: Jalisco, México, 2015; Volume 1, pp. 343–378. [Google Scholar]
- Martín, C.; Marcet, M.; Almazán, O.; Jönsson, L.J. Adaptation of a Recombinant Xylose-Utilizing Saccharomyces Cerevisiae Strain to a Sugarcane Bagasse Hydrolysate with High Content of Fermentation Inhibitors. Bioresour. Technol. 2007, 98, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez-Covarrubias, G.; Lange, S.E.; Rowell, R.M. Utilization of Byproducts from the Tequila Industry: Part 1: Agave Bagasse as a Raw Material for Animal Feeding and ®berboard Production. Bioresour. Technol. 2001, 77, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, H.M.; Chanona-Pérez, J.J.; Vega, A.; Ligero, P.; Mendoza-Pérez, J.A.; Calderón-Domínguez, G.; Terrés, E.; Farrera-Rebollo, R.R. Acetosolv Treatment of Fibers from Waste Agave Leaves: Influence of Process Variables and Microstructural Study. Ind. Crops Prod. 2016, 86, 163–172. [Google Scholar] [CrossRef]
- Caspeta, L.; Caro-Bermúdez, M.A.; Ponce-Noyola, T.; Martinez, A. Enzymatic Hydrolysis at High-Solids Loadings for the Conversion of Agave Bagasse to Fuel Ethanol. Appl. Energy 2014, 113, 277–286. [Google Scholar] [CrossRef]
- Saucedo-Luna, J.; Castro-Montoya, A.J.; Martinez-Pacheco, M.M.; Sosa-Aguirre, C.R.; Campos-Garcia, J. Efficient Chemical and Enzymatic Saccharification of the Lignocellulosic Residue from Agave Tequilana Bagasse to Produce Ethanol by Pichia Caribbica. J. Ind. Microbiol. Biotechnol. 2011, 38, 725–732. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Rangel-Mendez, J.R. Production of Activated Carbon from Organic By-Products from the Alcoholic Beverage Industry: Surface Area and Hardness Optimization by Using the Response Surface Methodology. Ind Crops Prod. 2011, 34, 1528–1537. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Rangel-Mendez, J.R. In Situ Transformation of Agave Bagasse into Activated Carbon by Use of an Environmental Scanning Electron Microscope. Microporous Mesoporous Mater. 2013, 167, 249–253. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Terrones, M.; Rangel-Mendez, J.R. Development of Highly Microporous Activated Carbon from the Alcoholic Beverage Industry Organic By-Products. Biomass Bioenergy 2011, 35, 103–112. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Hinojosa, M. Bagasse from the Mezcal Industry as an Alternative Renewable Energy Produced in Arid Lands. Fuel 2010, 89, 4049–4052. [Google Scholar] [CrossRef]
- International Standard Organization. ISO 14040: Environmental Management-Life Cycle Assessment Principles and Framework; International Standard Organization: Geneva, Switzerland, 1997. [Google Scholar]
- International Standard Organization. ISO 14044: Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Standard Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Chiew, C.S.C.; Gourich, W.; Pasbakhsh, P.; Poh, P.E.; Tey, B.T.; Song, C.P.; Chan, E.S. Life Cycle Assessment on Alginate-Based Nanocomposite Beads for the Removal of Lead(II) from Aqueous Solutions. J. Water Process. Eng. 2022, 45, 102531. [Google Scholar] [CrossRef]
- Nishikawa, E.; da Silva, M.G.C.; Vieira, M.G.A. Cadmium Biosorption by Alginate Extraction Waste and Process Overview in Life Cycle Assessment Context. J. Clean. Prod. 2018, 178, 166–175. [Google Scholar] [CrossRef]
- Kazemi, A.; Bahramifar, N.; Heydari, A.; Olsen, S.I. Life Cycle Assessment of Nanoadsorbents at Early Stage Technological Development. J. Clean. Prod. 2018, 174, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Yami, T.L.; Du, J.; Brunson, L.R.; Chamberlain, J.F.; Sabatini, D.A.; Butler, E.C. Life Cycle Assessment of Adsorbents for Fluoride Removal from Drinking Water in East Africa. Int. J. Life Cycle Assess. 2015, 20, 1277–1286. [Google Scholar] [CrossRef]
- de Hoces, M.C.; Vicente, I.D.L.C.A.; Rico, I.L.R.; Falcón, M.F.C.; Martín-Lara, M.Á. Life Cycle Assessment on Producing a Heavy Metals Biosorbent from Sugarcane Bagasse. Desalination Water Treat. 2011, 30, 272–277. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; del Olmo-Iruela, M.; Carrasco-Marín, F.; González-Gutiérrez, L.V. Amino-Functionalized Material from a Bio-Template for Silver Adsorption: Process Evaluation in Batch and Fixed Bed. J. Chem. Technol. Biotechnol. 2018, 94, 590–599. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Xia, M.; Wang, F.; Ju, X. Three-Dimension Hierarchical Composite via in-Situ Growth of Zn/Al Layered Double Hydroxide Plates onto Polyaniline-Wrapped Carbon Sphere for Efficient Naproxen Removal. J. Hazard. Mater. 2022, 423, 127192. [Google Scholar] [CrossRef]
- Díaz-Blancas, V.; Aguilar-Madera, C.G.; Flores-Cano, J.V.; Leyva-Ramos, R.; Padilla-Ortega, E.; Ocampo-Pérez, R. Evaluation of Mass Transfer Mechanisms Involved during the Adsorption of Metronidazole on Granular Activated Carbon in Fixed Bed Column. J. Water Process. Eng. 2020, 36, 101303. [Google Scholar] [CrossRef]
- Margni, M.; Curran, M.A. 4 Life Cycle Impact Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bessadok, A.; Langevin, D.; Gouanvé, F.; Chappey, C.; Roudesli, S.; Marais, S. Study of Water Sorption on Modified Agave Fibres. Carbohydr. Polym. 2009, 76, 74–85. [Google Scholar] [CrossRef]
- Kestur, G.S.; Flores-Sahagun, T.H.S.; dos Santos, L.P.; dos Santos, J.; Mazzaro, I.; Mikowski, A. Characterization of Blue Agave Bagasse Fibers of Mexico. Compos. Part. A Appl. Sci. Manuf. 2013, 45, 153–161. [Google Scholar] [CrossRef]
- Singha, A.S.; Rana, R.K. Functionalization of Cellulosic Fibers by Graft Copolymerization of Acrylonitrile and Ethyl Acrylate from Their Binary Mixtures. Carbohydr. Polym. 2012, 87, 500–511. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.N.L.; Cavalcante, D.D.; da Silva, E.G.P.; das Virgens, C.F.; Dias, F.D.S. Biosorption of Pb(II) and Cd(II) Ions by Agave Sisalana (Sisal Fiber). Microchem. J. 2011, 97, 269–273. [Google Scholar] [CrossRef]
- el Oudiani, A.; Msahli, S.; Sakli, F. In-Depth Study of Agave Fiber Structure Using Fourier Transform Infrared Spectroscopy. Carbohydr. Polym. 2017, 164, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Ronda, A.; della Zassa, M.; Martín-Lara, M.A.; Calero, M.; Canu, P. Combustion of a Pb(II)-Loaded Olive Tree Pruning Used as Biosorbent. J. Hazard. Mater. 2016, 308, 285–293. [Google Scholar] [CrossRef]
- Vlaev, L.; Nedelchev, N.; Gyurova, K.; Zagorcheva, M. A Comparative Study of Non-Isothermal Kinetics of Decomposition of Calcium Oxalate Monohydrate. J. Anal. Appl. Pyrolysis 2008, 81, 253–262. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and Characterization of Hydrochar from Waste Eucalyptus Bark by Hydrothermal Carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Jeder, A.; Sanchez-Sanchez, A.; Gadonneix, P.; Masson, E.; Ouederni, A.; Celzard, A.; Fierro, V. The Severity Factor as a Useful Tool for Producing Hydrochars and Derived Carbon Materials. Environ. Sci. Pollut. Res. 2018, 25, 1497–1507. [Google Scholar] [CrossRef]
- Das, D.; Mukherjee, M.; Pal, A.K.; Ghosh, A.K. Extraction of Xylem Fibers from Musa Sapientum and Characterization. Fibers Polym. 2017, 18, 2225–2234. [Google Scholar] [CrossRef]
- Truss, R.W.; Wood, B.; Rasch, R. Quantitative Surface Analysis of Hemp Fibers Using XPS, Conventional and Low Voltage in-Lens SEM. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Vickers, P.E.; Baillie, C.A.; Watts, J.F. An Experimental Investigation of Modified and Unmodified Flax Fibres with XPS, ToF-SIMS and ATR-FTIR. J. Mater. Sci 2003, 38, 3903–3914. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized Adsorbents Prepared from Fruit Peels: Equilibrium, Kinetic and Thermodynamic Studies for Copper Adsorption in Aqueous Solution. J. Clean Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Joshiba, G.J.; Kumar, P.S.; Govarthanan, M.; Ngueagni, P.T.; Abilarasu, A.; Carolin, C.F. Investigation of Magnetic Silica Nanocomposite Immobilized Pseudomonas Fluorescens as a Biosorbent for the Effective Sequestration of Rhodamine B from Aqueous Systems. Environ. Pollut. 2021, 269. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, H.; Senthil Kumar, P.; Seenuvasan, M.; Anil Kumar, M. Kinetics, Equilibrium and Thermodynamic Investigations of Methylene Blue Dye Removal Using Casuarina Equisetifolia Pines. Chemosphere 2021, 285. [Google Scholar] [CrossRef]
- Mohammad-Rezaei, R.; Khalilzadeh, B.; Rahimi, F.; Moradi, S.; Shahlaei, M.; Derakhshankhah, H.; Jaymand, M. Simultaneous Removal of Cationic and Anionic Dyes from Simulated Industrial Effluents Using a Nature-Inspired Adsorbent. Environ. Res. 2022, 214. [Google Scholar] [CrossRef] [PubMed]
- Castellar-Ortega, G.; Mendoza, E.; Angulo, E.; Paula, Z.; Rosso, M.; Jaramillo, J. Equilibrium, Kinetic and Thermodynamic of Direct Blue 86 Dye Adsorption on Activated Carbon Obtained from Manioc Husk. Rev. MVZ Cordoba 2019, 24, 7231–7238. [Google Scholar] [CrossRef] [Green Version]
- El Nemr, A.; Abdelwahab, O.; El-Sikaily, A.; Khaled, A. Removal of Direct Blue-86 from Aqueous Solution by New Activated Carbon Developed from Orange Peel. J. Hazard. Mater. 2009, 161, 102–110. [Google Scholar] [CrossRef]
- Garg, D.; Majumder, C.B.; Kumar, S.; Sarkar, B. Removal of Direct Blue-86 Dye from Aqueous Solution Using Alginate Encapsulated Activated Carbon (PnsAC-Alginate) Prepared from Waste Peanut Shell. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Li, Y.P.; Xiang, P.; Chen, H.T.; Zhou, Y. Adsorption Performance of One- and Two-Component Anionic Dyes Using Core-Shell ZIF-8@ZIF-67. J. Solid State Chem. 2022, 315. [Google Scholar] [CrossRef]
- Ghariani, B.; Hadrich, B.; Louati, I.; Mtibaa, R.; Daassi, D.; Rodriguez-Couto, S.; Nasri, M.; Mechichi, T. Porous Heat-Treated Fungal Biomass: Preparation, Characterization and Application for Removal of Textile Dyes from Aqueous Solutions. J. Porous Mater. 2019, 26, 1475–1488. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Aguilar-Armenta, G.; Gonzalez-Gutierrez, L.V.; Guerrero-Coronado, R.M.; Mendoza-Barron, J. Ammonia Exchange on Clinoptilolite from Mineral Deposits Located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Menyar, A.; Hamissa, B.; Brouers, F.; Mahjoub, B.; Seffen, M. Adsorption of Textile Dyes Using Agave Americana (L.) Fibres: Equilibrium and Kinetics Modelling. Adsorpt. Sci. Technol. 2007, 25, 311–325. [Google Scholar]
- Benjedim, S.; Romero-Cano, L.A.; Pérez-Cadenas, A.F.; Bautista-Toledo, M.I.; Lotfi, E.M.; Carrasco-Marín, F. Removal of Emerging Pollutants Present in Water Using an E-Coli Biofilm Supported onto Activated Carbons Prepared from Argan Wastes: Adsorption Studies in Batch and Fixed Bed. Sci. Total Environ. 2020, 720. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; González-Gutiérrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Grapefruit Peels as Biosorbent: Characterization and Use in Batch and Fixed Bed Column for Cu(II) Uptake from Wastewater. J. Chem. Technol. Biotechnol. 2017, 92, 1650–1658. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan-Clay Composite as Highly Effective and Low-Cost Adsorbent for Batch and Fixed-Bed Adsorption of Methylene Blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Coalesced Chitosan Activated Carbon Composite for Batch and Fixed-Bed Adsorption of Cationic and Anionic Dyes. Colloids Surf. B Biointerfaces 2013, 105, 199–206. [Google Scholar] [CrossRef]
- Das Saha, P.; Chakraborty, S.; Chowdhury, S. Batch and Continuous (Fixed-Bed Column) Biosorption of Crystal Violet by Artocarpus Heterophyllus (Jackfruit) Leaf Powder. Colloids Surf. B Biointerfaces 2012, 92, 262–270. [Google Scholar] [CrossRef]
- Varadarajan, G.; Venkatachalam, P. Sustainable Textile Dyeing Processes. Environ. Chem. Lett. 2016, 14, 113–122. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Calculador de Equivalencias de Gases de Efecto Invernadero. United States Government (n.d.). Available online: https://espanol.epa.gov/ (accessed on 1 November 2022).








| Temperature Range (°C) | Composition | (%) |
|---|---|---|
| 20–140 | humidity and water | 2.35 |
| 140–227 | hemicellulose | 3.35 |
| 227–400 | cellulose | 40.94 |
| 400–580 | lignin | 20.15 |
| 580–790 | CaCO3 decomposition | 15.73 |
| 790–950 | inorganic compounds | 17.47 |
| C1s | Bond | FWHM (eV) | % Peak | O1s | Bond | % Peak | %O | N1s | Bond | % Peak | %N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 284.79 | C-C | 1.52 | 58.07 | 531.80 | C=O | 29.26 | 18.28 | 398.10 | Amide | 6.84 | 0.54 |
| 286.40 | C-O | 31.59 | 533.00 | C-O | 58.27 | 400.10 | Amide | 83.98 | |||
| 287.90 | C=O | 7.39 | 542.20 | O-H | 12.46 | 402.10 | NH3+ | 9.17 | |||
| 289.00 | O-C=O | 2.93 |
| Material | T | qe, exp | Pseudo First Order | Pseudo Second Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe, calc | R | k2 | qe, calc | R | a | b | R | |||
| (°C) | (mg g−1) | (min −1) | (mg g−1) | (g mg−1 min−1) | (mg g−1) | ||||||
| Agave fiber | 25 | 2.80 | 0.0174 | 2.71 | 0.9778 | 0.0061 | 3.23 | 0.9911 | 3.103 | 0.0489 | 0.9971 |
| 35 | 2.80 | 0.0099 | 2.73 | 0.9809 | 0.0026 | 3.55 | 0.9878 | 2.312 | 0.0193 | 0.9930 | |
| 45 | 2.70 | 0.0128 | 2.53 | 0.9562 | 0.0045 | 3.10 | 0.9733 | 3.000 | 0.0301 | 0.9861 | |
| Hydrochar | 25 | 2.60 | 0.0173 | 2.52 | 0.9762 | 0.0066 | 3.01 | 0.9898 | 3.339 | 0.0455 | 0.9965 |
| 35 | 2.60 | 0.0104 | 2.55 | 0.9814 | 0.0030 | 3.30 | 0.9885 | 2.513 | 0.0190 | 0.9936 | |
| 45 | 2.51 | 0.0136 | 2.35 | 0.9587 | 0.0050 | 2.88 | 0.9755 | 3.249 | 0.0285 | 0.9879 | |
| Material | T | qmax exp | Langmuir Model | Freundlich Model | Temkin Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | K | R | Kf | n | R | AT | bT | R | |||
| (°C) | (mg g−1) | (mg g−1) | (L mg g−1) | (L mg−1) | (J mol−1) | ||||||
| Agave fibers | 25 | 5.91 | 6.29 | 0.022 | 0.990 | 1.19 | 3.79 | 0.997 | 0.393 | 2193.50 | 0.992 |
| 35 | 6.24 | 6.56 | 0.023 | 0.996 | 1.30 | 3.90 | 0.997 | 0.396 | 2158.64 | 0.998 | |
| 45 | 6.49 | 6.57 | 0.025 | 0.990 | 1.37 | 4.02 | 0.985 | 0.416 | 2226.42 | 0.963 | |
| Hydrochar | 25 | 5.49 | 5.83 | 0.024 | 0.991 | 1.13 | 3.81 | 0.998 | 0.422 | 2358.60 | 0.992 |
| 35 | 5.80 | 6.10 | 0.025 | 0.996 | 1.23 | 3.91 | 0.997 | 0.426 | 2321.17 | 0.998 | |
| 45 | 6.03 | 6.12 | 0.027 | 0.990 | 1.30 | 4.02 | 0.986 | 0.447 | 2394.00 | 0.963 | |
| Material | q(max) (mg g−1) | Reference |
|---|---|---|
| Biosorbent (Agave Fibers) | 6.57 | Present Work |
| Manioc husk activated carbon | 6.20 | [41] |
| Merck commercial activated carbon | 3.70 | |
| Orange peel activated carbon | 33.78 | [42] |
| Peanut shell activated carbon | 21.60 | [43] |
| Zeolitic Imidazolate Frameworks (ZIF-8) | 128.00 | [44] |
| Zeolitic Imidazolate Frameworks (ZIF-67) | 230.00 | |
| Zeolitic Imidazolate Frameworks (ZIF-8@ZIF-6) | 301.69 | |
| Granular particles of heat treated-lyophilized biomass of Neonectria radicicola | 227.1 | [45] |
| C0 | qs | qb | Tb | HMZT | Adams-Bohart | Thomas | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| KAB | N0 | R | Kth | q0 | R | |||||
| mg L−1 | mg g−1 | mg g−1 | min | cm | L mg−1 min−1 | mg L−1 | mL mg−1 min−1 | mg g−1 | ||
| 5 | 13.58 | 0.00 | --- | 1.00 | 1.15 × 10−5 | 8.58 × 104 | 0.6653 | 1.03 | 8.64 | 0.9678 |
| 10 | 23.18 | 0.69 | 5 | 0.96 | 3.37 × 10−5 | 4.58 × 104 | 0.8032 | 0.44 | 18.91 | 0.9736 |
| 25 | 18.99 | 4.96 | 125 | 0.73 | 1.44 × 10−4 | 2.01 × 104 | 0.8842 | 0.72 | 22.90 | 0.9943 |
| 50 | 15.23 | 7.22 | 240 | 0.75 | 4.39 × 10−4 | 1.06 × 104 | 0.9144 | 1.66 | 15.85 | 0.9980 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Navarro, C.S.; Warren-Vega, W.M.; Serna-Carrizales, J.C.; Zárate-Guzmán, A.I.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Collins-Martínez, V.H.; Niembro-García, J.; Romero-Cano, L.A. Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry. Materials 2023, 16, 8. https://doi.org/10.3390/ma16010008
Gómez-Navarro CS, Warren-Vega WM, Serna-Carrizales JC, Zárate-Guzmán AI, Ocampo-Pérez R, Carrasco-Marín F, Collins-Martínez VH, Niembro-García J, Romero-Cano LA. Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry. Materials. 2023; 16(1):8. https://doi.org/10.3390/ma16010008
Chicago/Turabian StyleGómez-Navarro, Camila S., Walter M. Warren-Vega, Juan C. Serna-Carrizales, Ana I. Zárate-Guzmán, Raúl Ocampo-Pérez, Francisco Carrasco-Marín, Virginia H. Collins-Martínez, Joaquina Niembro-García, and Luis A. Romero-Cano. 2023. "Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry" Materials 16, no. 1: 8. https://doi.org/10.3390/ma16010008
APA StyleGómez-Navarro, C. S., Warren-Vega, W. M., Serna-Carrizales, J. C., Zárate-Guzmán, A. I., Ocampo-Pérez, R., Carrasco-Marín, F., Collins-Martínez, V. H., Niembro-García, J., & Romero-Cano, L. A. (2023). Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry. Materials, 16(1), 8. https://doi.org/10.3390/ma16010008