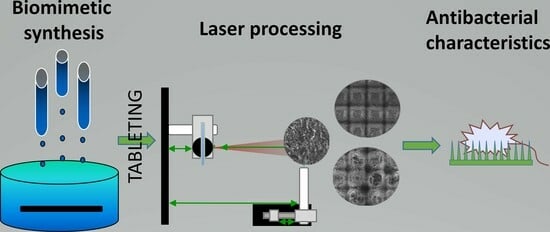
Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mg/Zn Double-Doped β-TCP and Preparation of Ceramic Tablets
2.2. Processing with Femtosecond Laser
2.3. Characterization
2.3.1. Chemical Analysis
2.3.2. XRD Analysis
2.3.3. Confocal Microscopy
2.3.4. Raman Spectroscopy
2.3.5. Scanning Electron Microscopy (SEM) Analysis of Tablet Morphology and Microstructure
2.3.6. Estimation of Bacterial Viability by Plate Count
2.3.7. Evaluation of E. coli Cell Morphology by Scanning Electron Microscopy (SEM)
3. Results
3.1. Synthesis of Mg/Zn Double-Doped β-TCP and Preparation of Ceramic Tablets
3.2. Processing with Femtosecond Laser
3.3. Analysis of an Antimicrobial Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphate (CAPO4) scaffolds for bone tissue engineering applications. J. Biotechnol. Biomed. Sci. 2018, 1, 25–93. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4)-Based Bioceramics: Preparation, Properties, and Applications. Coatings 2022, 12, 1380. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Oshita, M.; Kataoka, M.; Azuma, Y.; Furuzono, T. Shareability of antibacterial and osteoblastic-proliferation activities of zinc-doped hydroxyapatite nanoparticles in vitro. J. Biomed. Mater. Res. B Appl. 2022, 110, 799. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, D.; Gopi, D.; Ramya, S.; Kavitha, L. Investigation of anticorrosive, antibacterial and in vitro biological properties of a sulphonated poly(etheretherketone)/strontium, cerium co-substituted hydroxyapatite composite coating developed on surface treated surgical grade stainless steel for orthopedic applications. RSC Adv. 2014, 4, 61525–61536. [Google Scholar] [CrossRef]
- Yang, N.; Wang, S.; Ding, P.; Sun, S.; Wei, Q.; Jafari, H.; Wang, L.; Han, Y.; Valentine, O.O.; Wang, T.; et al. Magnesium-doped biphasic calcium phosphate nanoparticles with incorporation of silver: Synthesis, cytotoxic and antibacterial property. Mater. Lett. 2022, 322, 132478. [Google Scholar] [CrossRef]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; de Bruijn, J.D.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Brito, A.; Brazete, D.; Pereira, I.; Carrilho, E.; Abrantes, A.; Pires, A.; Aguiar, M.; Carvalho, L.; Botelho, M.; et al. Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats. Materials 2019, 12, 4. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882. [Google Scholar] [CrossRef]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1426. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, M.N.; Akdas, S.; Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol. Trace Elem. Res. 2021, 199, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Kuo, H.-C.; Syu, M.-L.; Tuan, W.-H.; Lai, P.-L. A head-to-head comparison of the degradation rate of resorbable bioceramics. Mater. Sci. Eng. C 2020, 106, 110175. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C.; Eanes, E.D. Octacalcium Phosphate, 1st ed.; S Karger Ag: Basel, Switzerland, 2001. [Google Scholar]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rigotti, R.L.; Tardelli, J.D.C.; dos Reis, A.C. Influence of antibacterial surface treatment on dental implants on cell viability: A systematic review. Heliyon 2023, 9, e13693. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Parsikia, F.; Amini, P.; Asgari, S. Influence of mechanical and chemical surface treatments on the formation of bonelike structure in cpTi for endosseous dental implants. Appl. Surf. Sci. 2012, 259, 283–287. [Google Scholar] [CrossRef]
- Kubo, A.L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and Antiviral Effects of Ag, Cu and Zn Metals, Respective Nanoparticles and Filter Materials Thereof against Coronavirus SARS-CoV-2 and Influenza A Virus. Pharmaceutics 2022, 14, 2549. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved. Acta Biomater. 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Trifonov, A.; Lasgorceix, M.; Hocquet, S.; Minne, M.; Declercq, H.; Leriche, A.; Aceti, D.; Buchvarov, I. Development of Femtosecond Laser-Engineered β-Tricalcium Phosphate (β-TCP) Biomimetic Templates for Orthopaedic Tissue Engineering. Appl. Sci. 2021, 11, 2565. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Carvalho, A.; Trifonov, A.; Nathala, C.; Monteiro, F.; Buchvarov, I. Effect of surface modification by femtosecond laser on zirconia based ceramics for screening of cell-surface interaction. Appl. Surf. Sci. 2020, 513, 145914. [Google Scholar] [CrossRef]
- Faria, D.; Henriques, B.; Souza, A.C.; Silva, F.S.; Carvalho, O. Laser-assisted production of HAp-coated zirconia structured surfaces for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 112, 104049. [Google Scholar] [CrossRef] [PubMed]
- Lutey, A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef] [PubMed]
- Dyukin, R.; Martsinovskiy, G.; Sergaeva, O.; Shandybina, G.; Svirina, V.; Yakovlev, E. Interaction of Femtosecond Laser Pulses with Solids: Electron/Phonon/Plasmon Dynamics. In Laser Pulses—Theory, Technology, and Applications; Peshko, I., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast laser processing of materials: From science to industry. Light Sci. Appl. 2016, 5, e1613. [Google Scholar] [CrossRef] [PubMed]
- Stanciuc, A.; Flamant, Q.; Sprecher, C.M.; Alini, M.; Anglada, M.; Peroglio, M. Femtosecond laser multi-patterning of zirconia for screening of cell-surface interactions. J. Eur. Ceram. 2018, 38, 939–948. [Google Scholar] [CrossRef]
- Buchberger, G.; Muck, M.; Plamadeala, C.; Heitz, J. Laser Structuring for Biomedical Applications. In Laser Pulses—Theory, Technology, and Applications; Peshko, I., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Zhang, Y.; Ito, Y.; Sun, H.; Sugita, S. Investigation of multi-timescale processing phenomena in femtosecond laser drilling of zirconia ceramics. Opt. Express 2022, 30, 37394–37406. [Google Scholar] [CrossRef]
- Chen, B.C.; Tsai, Y.; Ho, C.; Chen, C.; Ma, C. Parametric effects on femtosecond laser ablation of Al2O3 ceramics. Ceram. Int. 2013, 39, S341–S344. [Google Scholar] [CrossRef]
- Kokubo, T.J. Surface chemistry of bioactive glass-ceramics. Non-Cryst. Solids 1990, 120, 138–151. [Google Scholar] [CrossRef]
- ISO 4287:1997; Geometrical Product Specifications (GPS)—Surface texture: Profile method—Terms, definitions and surface texture parameters. ISO: Geneva, Switzerland, 1997.
- Sjollema, J.; Zaat, S.A.J.; Fontaine, V.; Ramstedt, M.; Luginbuehl, R.; Thevissen, K.; Li, J.; van der Mei, H.C.; Busscher, H.J. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018, 70, 12–24. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal Structure Analysis of β-Tricalcium Phosphate Ca3(PO4)2 by Neutron Powder Diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Zaichick, V. Data for the Reference Man: Skeleton content of chemical elements. Radiat. Environ. Biophys. 2013, 52, 65–85. [Google Scholar] [CrossRef]
- Andonova-Lilova, B.; Alexandrova, R.; Rabadjieva, D.; Tepavitcharova, S. Application of cultured murine cells for initial evaluation of the biocompatibility of Mg and Zn-modified tri-calcium phosphates. Compt. Rend. Acad. Bulg. Sci. 2012, 65, 1099. [Google Scholar]
- Rabadjieva, D.; Tepavitcharova, S.; Sezanova, K.; Gergulova, R.; Titorenkova, R.; Petrov, O.; Dyulgerova, E.B. Biomimetic modifications of calcium orthophosphates. In On Biomimetic, 1st ed.; Pramatarova, L., Ed.; InTech: Rijeka, Croatia, 2011; pp. 135–162. [Google Scholar]
- Rabadjieva, D.; Titorenkova, R.; Gergulova, R.; Dyulgerova, E.; Balarew, C. Influence of Zn on the Biomimetic Transformation of Amorphous Calcium Phosphate to Nano-Sized Apatite. Nanosci. Nanotechnol. 2009, 9, 235–238. [Google Scholar]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Rabadjieva, D.; Tepavitcharova, S.; Gergulova, R.; Sezanova, K.; Titorenkova, R.; Petrov, O.; Dyulgerova, E. Mg- and Zn-modified calcium phosphates prepared by biomimetic precipitation and subsequent treatment at high temperature. J. Mater. Sci. Mater. Med. 2011, 22, 2187. [Google Scholar] [CrossRef]








| Components | SBFc [32] | Solution 1 | Solution 2 | Solution 3 | Buffer Solution |
|---|---|---|---|---|---|
| Na+ | 141.9 | 141.9 | 141.9 | 141.9 | 100 |
| K+ | 3.0 | 501 | 3 | 3 | |
| Mg2+ | 1.5 | 0 | 58.4 | 1.5 | |
| Ca2+ | 2.5 | 0 | 374 | 2.5 | |
| Zn2+ | 0 | 0 | 0 | 12.2 | |
| Cl− | 143 | 143 | 1008 | 167 | 97.5 |
| SO42− | 0.5 | 0.5 | 0.5 | 0.5 | |
| HCO3− | 4.2 | 4.2 | 4.2 | 4.2 | |
| HPO42− | 1.0 | 250 | 0 | 0 | |
| Valine | 512 | 512 | 512 | 512 | |
| pH | 7.2–7.4 | 8.0–8.2 | 8.0–8.2 | 6.5 | 8.0–8.2 |
| Sample | a [Å] | c [Å] | V, [Å3] |
|---|---|---|---|
| β-TCP [35] | 10.4352 (2) | 37.4029 (5) | 3482 |
| This study | 10.3238 (1) | 37.2624 (6) | 3439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalova, A.; Sezanova, K.; Angelova, L.; Paunova-Krasteva, T.; Gergulova, R.; Kovacheva, D.; Rabadjieva, D. Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity. Materials 2023, 16, 6626. https://doi.org/10.3390/ma16206626
Daskalova A, Sezanova K, Angelova L, Paunova-Krasteva T, Gergulova R, Kovacheva D, Rabadjieva D. Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity. Materials. 2023; 16(20):6626. https://doi.org/10.3390/ma16206626
Chicago/Turabian StyleDaskalova, Albena, Kostadinka Sezanova, Liliya Angelova, Tsvetelina Paunova-Krasteva, Rumiana Gergulova, Daniela Kovacheva, and Diana Rabadjieva. 2023. "Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity" Materials 16, no. 20: 6626. https://doi.org/10.3390/ma16206626
APA StyleDaskalova, A., Sezanova, K., Angelova, L., Paunova-Krasteva, T., Gergulova, R., Kovacheva, D., & Rabadjieva, D. (2023). Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity. Materials, 16(20), 6626. https://doi.org/10.3390/ma16206626