Mg, Zn Substituted Calcium Phosphates—Thermodynamic Modeling, Biomimetic Synthesis in the Presence of Low-Weight Amino Acids and High Temperature Properties
Abstract
:1. Introduction
2. Materials and Methods
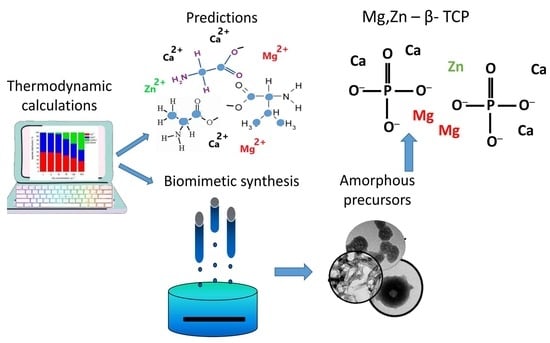
2.1. Thermodynamic Calculations
2.2. Biomimetic Synthesis of (Mg, Zn)-Doped Calcium Phosphates
2.2.1. Initial Solutions
2.2.2. Precipitation Method
2.3. Calcination of (Mg, Zn)-Doped Calcium Phosphates
2.4. Characterization
2.4.1. Chemical Analysis
2.4.2. Infrared Spectroscopy (IR)
2.4.3. X-ray Diffraction Analysis (XRD)
2.4.4. Transition Electron Microscopy (TEM) Analysis
2.4.5. Measurement of Specific Surface Area
3. Results
3.1. Thermodynamic Modeling
3.2. Biomimetic Synthesis
3.3. High Temperature Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Zhou, Y.; Lin, X.; Li, H. Macrophage Polarization Related to Biomimetic Calcium Phosphate Coatings: A Preliminary Study. Materials 2023, 16, 332. [Google Scholar] [CrossRef] [PubMed]
- Kulhánková, J.; Rohanová, D.; Horkavcová, D.; Bezdička, P.; Boccaccini, A.R. The role of (HCO3)− ions in SBF on the interaction with bioactive glass-ceramic scaffold. Mater. Today Chem. 2023, 28, 101367. [Google Scholar] [CrossRef]
- Canepa, A.; Filho, J.C.; Gutierrez, A.; Carrea, A.; Forsberg, A.M.; Nilsson, E.; Verrina, E.; Perfumo, F.; Bergstrom, J. Free amino acids in plasma, red blood cells, polymorphonuclear leukocytes, and muscle in normal and uraemic children. Nephrol. Dial. Transplant. 2002, 17, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Rautaray, D.; Mandal, S.; Sastry, M. Synthesis of Hydroxyapatite Crystals Using Amino Acid-Capped Gold Nanoparticles as a Scaffold. Langmuir 2005, 21, 5185–5191. [Google Scholar] [CrossRef] [PubMed]
- Jack, K.S.; Vizcarra, T.G.; Trau, M. Characterization and Surface Properties of Amino-Acid-Modified Carbonate-Containing Hydroxyapatite Particles. Langmuir 2007, 23, 12233–12242. [Google Scholar] [CrossRef]
- Palazzo, B.; Walsh, D.; Iafisco, M.; Foresti, E.; Bertinetti, L.; Martra, G.; Bianchi, C.L.; Cappelletti, G.; Roveri, N. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals. Acta Biomater. 2009, 5, 1241–1252. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Yao, G.; Cerruti, M. The importance of amino acid interactions in the crystallization of hydroxyapatite. J. R. Soc. Interface 2013, 10, 20120906. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Zhao, W.; Sahai, N. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 2015, 3, 9157–9167. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Zhang, Q.; Deng, X.; Yang, X.; et al. Oriented and Ordered Biomimetic Remineralization of the Surface of Demineralized Dental Enamel Using HAP@ACP Nanoparticles Guided by Glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Zeng, Y.; Xu, X.; Tang, R. Roles of Amorphous Calcium Phosphate and Biological Additives in the Assembly of Hydroxyapatite Nanoparticles. J. Phys. Chem. B 2007, 111, 13410–13418. [Google Scholar] [CrossRef] [PubMed]
- Gerk, S.A.; Golovanova, O.A.; Odazhiu, V.N. Structural, Morphological, and Resorption Properties of Carbonate Hydroxyapatite Prepared in the Presence of Glycine. Inorg. Mater. 2018, 54, 305–314. [Google Scholar] [CrossRef]
- Okada, M.; Oshita, M.; Kataoka, M.; Azuma, Y.; Furuzono, T. Shareability of antibacterial and osteoblastic-proliferation activities of zinc-doped hydroxyapatite nanoparticles in vitro. J. Biomed. Mater. Res. B Appl. 2022, 110, 799–805. [Google Scholar] [CrossRef]
- Tamay, D.G.; Gokyer, S.; Schmidt, J.; Vladescu, A.; Huri, P.Y.; Hasirci, V.; Hasirci, N. Corrosion Resistance and Cytocompatibility of Magnesium–Calcium Alloys Modified with Zinc- or Gallium-Doped Calcium Phosphate Coatings. ACS Appl. Mater. Interfaces 2022, 14, 104–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, S.; Ding, P.; Sun, S.; Wei, Q.; Jafari, H.; Wang, L.; Han, Y.; Valentine, O.O.; Wang, T.; et al. Magnesium-doped biphasic calcium phosphate nanoparticles with incorporation of silver: Synthesis, cytotoxic and antibacterial properties. Mater. Lett. 2022, 322, 132478. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Ballouze, R.; Marahat, M.H.; Mohamad, S.; Saidin, N.A.; Kasim, S.R.; Ooi, J.P. Biocompatible magnesium-doped biphasic calcium phosphate for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1426–1435. [Google Scholar] [CrossRef]
- Andonova-Lilova, B.; Alexandrova, R.; Rabadjieva, D.; Tepavitcharova, S. Application of cultured murine cells for initial evaluation of the biocompatability of Mg and Zn-modified tri-calcium phosphates. Compt. Rend. Acad. Bulg. Sci. 2012, 65, 1099–1104. [Google Scholar]
- Fu, T.; Hu, X.; Yang, C. Impact response analysis of stiffened sandwich functionally graded porous materials doubly-curved shell with re-entrant honeycomb auxetic core. Appl. Math. Model. 2023, 124, 553–575. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, T. Dynamic response of the hybrid honeycomb sandwich panel with zero Poisson’s ratio under low-speed impact. Mech. Adv. Mater. Struct. 2023, 1–15. [Google Scholar] [CrossRef]
- Hidayat, T.; Fallah-Mehrjardi, A.; Abdeyazdan, H.; Shishin, H.D.; Shevchenko, M.; Hayes, P.C.; Jak, E. Integrated Experimental and Thermodynamic Modeling Study of the Pb-Fe-O-S-Si System: Effect of Temperature and p(SO2) on Slag-Matte-Metal-Tridymite Equilibria. Metall. Mater. Trans. B 2023, 54, 536–549. [Google Scholar] [CrossRef]
- López-Porfiri, P.; Gorgojo, P.; González-Miquel, M. Solubility study and thermodynamic modelling of succinic acid and fumaric acid in bio-based solvents. J. Mol. Liq. 2023, 369, 120836. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Gergulova, R.; Titorenkova, R.; Tepavitcharova, S.; Dyulgerova, E.; Balarew, C.; Petrov, O. Biomimetic transformations of amorphous calcium phosphate: Kinetic and thermodynamic studies. J. Mater. Sci. Mater. Med. 2010, 21, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Rabadjieva, D.; Sezanova, K.; Gergulova, R.; Titorenkova, R.; Tepavitcharova, S. Precipitation and phase transformation of dicalcium phosphate dihydrate in electrolyte solutions of simulated body fluids: Thermodynamic modeling and kinetic studies. J. Biomed. Mater. Res. Part A 2020, 108, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations (US Geological Survey Techniques and Methods 2013). Available online: https://pubs.usgs.gov/tm/06/a43/ (accessed on 23 January 2013).
- Martell, A.E.; Smith, R.M.; Motekaitis, R.J. NIST Critically Selected Stability Constants of Metal Complexes Database; Texas A & M University: College Station, TX, USA, 2004. [Google Scholar]
- Magalhaes, M.C.; de Aguiar Pereira Marques, P.A.; Correi, R. Biomineralization-Medical Aspects of Solubility; Königsberger, E., Königsberger, L.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 71–123. [Google Scholar]
- Sovago, I.; Kiss, T.; Gergely, A. Critical survey of the stability constants of complexes of aliphatic amino acids. Pure Appl. Chem. 1993, 65, 1029–1080. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Tomashevsky, I.A. Kinetics and Thermodynamics of the Formation of Compounds of Calcium Ions and Amino Acids: Their Structure and Stability. Russ. J. Phys. Chem. A 2019, 93, 7–17. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Merck: Rahway, NJ, USA, 1989. [Google Scholar]
- Kokubo, T. Surface chemistry of bioactive glass-ceramics. J. Non-Cryst. Solids 1990, 120, 138–151. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Tepavitcharova, S.; Gergulova, R.; Sezanova, K.; Titorenkova, R.; Petrov, O.; Dyulgerova, E. Mg- and Zn-modified calcium phosphates prepared by biomimetic precipitation and subsequent treatment at high temperature. J. Mater. Sci. Mater. Med. 2011, 22, 2187–2196. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS V4 General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual Bruker AXS Karlsruhe. Germany. 2008. Available online: http//algol.fis.uc.pt/jap/TOPAS%204-2%20Users%20Manual.pdf (accessed on 15 January 2009).
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Betts, F.; Blumenthal, N.C.; Posner, A.S.; Becker, G.L.; Lehninger, A.L. Atomic structure of intracellular amorphous calcium phosphate deposits. Proc. Natl. Acad. Sci. USA 1975, 72, 2088–2090. [Google Scholar] [CrossRef]
- Zaichick, V. Data for the Reference Man: Skeleton content of chemical elements. Radiat. Environ. Biophys. 2013, 52, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Rabadjieva, D.; Tepavitcharova, S.; Sezanova, K.; Gergulova, R.; Titorenkova, R.; Petrov, O.; Dyulgerova, E.B. Biomimetic modifications of calcium orthophosphates. In On Biomimetic, 1st ed.; Pramatarova, L., Ed.; InTech: Rijeka, Croatia, 2011; pp. 135–162. [Google Scholar]
- Rabadjieva, D.; Titorenkova, R.; Gergulova, R.; Dyulgerova, E.; Balarew, C. Influence of Zn on the Biomimetic Transformation of Amorphous Calcium Phosphate to Nano-Sized Apatite. Nanosci. Nanotechnol. 2009, 9, 235–238. [Google Scholar]
- Martell, A.E.; Smith, R.M. (Eds.) NIST Standard Reference Database 46; Versions, 3, 4, 6, 7, 8; NIST: Gaithersburg, MD, USA, 2019.
- Plummer, L.N. Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2–H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3–CO2–H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Turner, D.R.; Whitfield, M.; Dickson, A.G. The equilibrium speciation of dissolved components in fresh water and sea water at 25 °C and 1 atm pressure. Geochim. Chosmochim. Acta 1981, 45, 855–881. [Google Scholar] [CrossRef]
- CEAM. US-EPA Minteq v. 3.11; US Environmental Protection Agency, CEAM: Athens, GA, USA, 1991.
- Ball, J.W.; Nordstrom, D.K. WATEQ4F-User’s Manual; Open-File Report 90-129; U.S. Geological Survey: Washington, DC, USA, 1991.
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium Phosphate Solubility Product from 4 to 37 °C. J. Res. Natl. Inst. Stand. Technol. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- McDowell, H.; Gregory, T.M.; Brown, W.E. Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37 °C. J. Res. Natl. Bur.Stand. Sect. A Phys. Chem. 1977, 81A, 273–281. [Google Scholar] [CrossRef]
- Christoffersen, M.R.; Christoffersen, J.; Kibalczyc, W. Apparent solubilities of two amorphous calcium phosphates and of octacalcium phosphate in the temperature range 30–42 °C. J. Cryst. Growth 1990, 106, 349–354. [Google Scholar] [CrossRef]
- Hamad, M.; Heughebaert, J.C. The growth of whitlockite. J. Cryst. Growth 1986, 79, 192–197. [Google Scholar] [CrossRef]







| Reaction | logK | Source |
|---|---|---|
| Mg3(PO4)2·8H2O = 3Mg2+ + 2PO43− + 8H2O | −25.20 | [27] |
| Mg3(PO4)2.22H2O = 3Mg2+ + 2PO43− + 22H2O | −23.30 | [27] |
| KMgPO4·6H2O = Mg2+ + K+ + PO43− + 6H2O | −10.62 | [27] |
| H+ + Ala− = H(Ala) | 9.72 | [28] |
| 2H+ + Ala− = H2(Ala)+ | 12.05 | [28] |
| H+ + Val− = H(Val) | 9.54 | [28] |
| 2H+ + Val− = H2(Val)+ | 11.82 | [28] |
| Ca2+ + Ala− = Ca(Ala)+ | 1.24 | [28] |
| Mg2+ + Ala− = Mg(Ala)+ | 1.96 | [28] |
| Zn2+ + Ala− = Zn(Ala)+ | 5.21 | [28] |
| Ca2+ + Val− = Ca(Val)+ | 1.02 | This study |
| Mg2+ + Val− = Mg(Val)+ | 1.72 | This study |
| Zn2+ + Val− = Zn(Val)+ | 5.00 | [28] |
| Components | SBFc [31] | Solution 1 | Solution 2 | Solution 3 | Buffer Solution |
|---|---|---|---|---|---|
| Na+ | 3.26 | 3.26 | 3.26 | 3.26 | 2.30 |
| K+ | 0.12 | 19.8 | 0.12 | 0.12 | |
| Mg2+ | 0.04 | 0.04 | 1.41 | 0.04 | |
| Ca2+ | 0.10 | 14.7 | 0.10 | ||
| Zn2+ | 0.86 | ||||
| Cl− | 5.07 | 5.07 | 26.2 | 6.03 | 3.46 |
| SO42− | 0.05 | 0.05 | 0.05 | 0.05 | |
| HCO3− | 0.26 | 0.78 | |||
| HPO42− | 0.10 | 24.1 | |||
| Gly/Ala/Val | |||||
| Series A | 7.5/7.5/7.5 | 7.5/7.5/7.5 | 7.5/7.5/7.5 | 7.5/7.5/7.5 | |
| Series B | 220/142/60 | 220/142/60 | 220/142/60 | 220/142/60 | |
| pH | 7.2–7.4 | 8.0–8.2 | 8.0–8.2 | 6.5 | 8.0 |
| Sample | CMg | CZn | (Ca2+ + Mg2+ + Zn2+)/P |
|---|---|---|---|
| mol% | Molar Ratio | ||
| Series A | |||
| CPGly7 | 7.63 | 2.61 | 1.54 |
| CPAla7 | 7.73 | 2.72 | 1.56 |
| CPVal7 | 7.91 | 2.82 | 1.56 |
| Series B | |||
| CPGly220 | 5.86 | 0.71 | 1.47 |
| CPAla142 | 6.37 | 1.73 | 1.54 |
| CPVal60 | 8.09 | 2.63 | 1.56 |
| Series A | Series B | ||
|---|---|---|---|
| CPGly7 | 39 | CPGly220 | 242 |
| CPAla7 | 37 | CPAla142 | 222 |
| CPVal7 | 49 | CPVal60 | 48 |
| Sample | CMg + CZn mol,% | a [Å] | c [Å] | Mean Size [nm] | V, [Å3] | Ca2P2O7 wt% |
|---|---|---|---|---|---|---|
| β—TCP [34] | 10.4352 (2) | 37.4029 (5) | 3482 | |||
| Series A | ||||||
| CPGly7 | 10.3 | 10.3240 (1) | 37.2646 (3) | 287 (1) | 3439 | 4.34 |
| CPAla7 | 10.5 | 10.3234 (1) | 37.2623 (6) | 280 (2) | 3439 | 3.58 |
| CPVal7 | 10.7 | 10.3211 (2) | 37.3006 (8) | 322 (1) | 3441 | 5.23 |
| Series B | ||||||
| CPGly220 | 6.57 | 10.3677 (1) | 37.2023 (3) | 372 (2) | 3463 | 3.87 |
| CPAla142 | 8.10 | 10.3503 (1) | 37.1326 (3) | 309 (5) | 3445 | 3.37 |
| CPVal60 | 10.7 | 10.3238 (1) | 37.2624 (6) | 276 (2) | 3439 | 1.44 |
| Samples | Ca(4) Position | Ca(5) Position | ||||
|---|---|---|---|---|---|---|
| Ca | Mg | Zn | Ca | Mg | Zn | |
| Series A | ||||||
| CPGly7 | 0.45 | 0.05 | 0.00 | 0.30 | 0.66 | 0.05 |
| CPAla7 | 0.29 | 0.07 | 0.14 | 0.08 | 0.76 | 0.15 |
| CPVal7 | 0.33 | 0.08 | 0.09 | 0.00 | 0.86 | 0.14 |
| Series B | ||||||
| CPGly220 | 0.37 | 0.10 | 0.02 | 0.24 | 0.62 | 0.13 |
| CPAla142 | 0.33 | 0.13 | 0.04 | 0.16 | 0.65 | 0.19 |
| CPVal60 | 0.43 | 0.07 | 0.00 | 0.06 | 0.76 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabadjieva, D.; Gergulova, R.; Sezanova, K.; Kovacheva, D.; Titorenkova, R. Mg, Zn Substituted Calcium Phosphates—Thermodynamic Modeling, Biomimetic Synthesis in the Presence of Low-Weight Amino Acids and High Temperature Properties. Materials 2023, 16, 6638. https://doi.org/10.3390/ma16206638
Rabadjieva D, Gergulova R, Sezanova K, Kovacheva D, Titorenkova R. Mg, Zn Substituted Calcium Phosphates—Thermodynamic Modeling, Biomimetic Synthesis in the Presence of Low-Weight Amino Acids and High Temperature Properties. Materials. 2023; 16(20):6638. https://doi.org/10.3390/ma16206638
Chicago/Turabian StyleRabadjieva, Diana, Rumiana Gergulova, Kostadinka Sezanova, Daniela Kovacheva, and Rositsa Titorenkova. 2023. "Mg, Zn Substituted Calcium Phosphates—Thermodynamic Modeling, Biomimetic Synthesis in the Presence of Low-Weight Amino Acids and High Temperature Properties" Materials 16, no. 20: 6638. https://doi.org/10.3390/ma16206638
APA StyleRabadjieva, D., Gergulova, R., Sezanova, K., Kovacheva, D., & Titorenkova, R. (2023). Mg, Zn Substituted Calcium Phosphates—Thermodynamic Modeling, Biomimetic Synthesis in the Presence of Low-Weight Amino Acids and High Temperature Properties. Materials, 16(20), 6638. https://doi.org/10.3390/ma16206638