Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
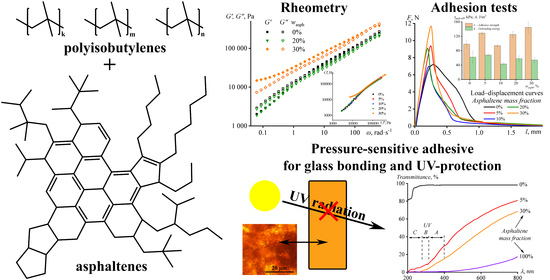
3.1. Rheology of Adhesives in the Hot State
3.2. Rheology of Adhesives at Normal Temperature
3.3. Adhesion Characteristics of Adhesives
3.4. Optical Properties of Adhesives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Fabo, E.C.; Noonan, F.P.; Frederick, J.E. Biologically effective doses of sunlight for immune suppression at various latitudes and their relationship to changes in stratospheric ozone. Photochem. Photobiol. 1990, 52, 811–817. [Google Scholar] [CrossRef]
- Baadsgaard, O. In vivo ultraviolet irradiation of human skin results in profound perturbation of the immune system: Relevance to ultraviolet-induced skin cancer. Arch. Dermatol. 1991, 127, 99–109. [Google Scholar] [CrossRef]
- Longstreth, J.D.; de Gruijl, F.R.; van der Leun, J.C.; Kripke, M.L.; Takizawa, Y. Effects of increased solar ultraviolet radiation on human health. Ambio 1995, 24, 153–165. [Google Scholar] [CrossRef]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Lim, H.W.; Gilchrest, B.A.; Cooper, K.D.; Bischoff-Ferrari, H.A.; Rigel, D.S.; Cyr, W.H.; Miller, S.; DeLeo, V.A.; Lee, T.K.; Demko, C.A.; et al. Sunlight, tanning booths, and vitamin D. J. Am. Acad. Dermatol. 2005, 52, 868–876. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Young, A.R.; Harrison, G.I.; Chadwick, C.A.; Nikaido, O.; Ramsden, J.; Potten, C.S. The Similarity of Action Spectra for Thymine Dimers in Human Epidermis and Erythema Suggests that DNA is the Chromophore for Erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef]
- Morimoto, T.; Tomonaga, H.; Mitani, A. Ultraviolet ray absorbing coatings on glass for automobiles. Thin Solid Film. 1999, 351, 61–65. [Google Scholar] [CrossRef]
- Vávrová, P.; Paulusová, H.; Kučerová, I. The Properties and Lifetime of Polymer UV Films. Restaurator 2004, 25, 233–248. [Google Scholar] [CrossRef]
- Kittas, C.; Tchamitchian, M.; Katsoulas, N.; Karaiskou, P.; Papaioannou, C. Effect of two UV-absorbing greenhouse-covering films on growth and yield of an eggplant soilless crop. Sci. Hortic. 2006, 110, 30–37. [Google Scholar] [CrossRef]
- Gugumus, F.; Klemchuk, P.; Pospisil, J. (Eds.) Inhibition of Oxidation Processes in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; Volume 2, Chapter 2. [Google Scholar]
- Gugumus, F. Plastics Additives Handbook, 5th ed.; Zweifel, H., Ed.; Hanser Publishers: Munich, Germany, 2001; Chapter 2. [Google Scholar]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Sakamoto, H.; Qiu, J.; Makishima, A. The preparation and properties of CeO2–TiO2film by sol–gel spin-coating process. Sci. Technol. Adv. Mater. 2003, 4, 69–76. [Google Scholar] [CrossRef]
- Kundu, D.; Mukherjee, R. UV absorbing transparent sol-gel derived coatings on glass. J. Mater. Sci. Lett. 2003, 22, 1647–1649. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H.; Rauch, K.; Dieckmann, U.; Nitsche, R.; Fritz, T. Optimized UV protecting coatings by combination of organic and inorganic UV absorbers. Thin Solid Film. 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Weichelt, F.; Emmler, R.; Flyunt, R.; Beyer, E.; Buchmeiser, M.R.; Beyer, M. ZnO-Based UV Nanocomposites for Wood Coatings in Outdoor Applications. Macromol. Mater. Eng. 2009, 295, 130–136. [Google Scholar] [CrossRef]
- Gugumus, F. Possibilities and limits of synergism with light stabilizers in polyolefins 2. UV absorbers in polyolefins. Polym. Degrad. Stab. 2002, 75, 309–320. [Google Scholar] [CrossRef]
- Maliakal, A.; Lem, G.; Turro, N.J.; Ravichandran, R.; Suhadolnik, J.C.; DeBellis, A.D.; Wood, M.G.; Lau, J. Twisted Intramolecular Charge Transfer States in 2-Arylbenzotriazoles: Fluorescence Deactivation via Intramolecular Electron Transfer Rather Than Proton Transfer. J. Phys. Chem. A 2002, 106, 7680–7689. [Google Scholar] [CrossRef]
- McGarry, P.F.; Jockusch, S.; Fujiwara, Y.; Kaprinidis, N.A.; Turro, N.J. DMSO Solvent Induced Photochemistry in Highly Photostable Compounds. The Role of Intermolecular Hydrogen Bonding. J. Phys. Chem. A 1997, 101, 764–767. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Hamid, S.H. Handbook of Polymer Degradation; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Petrukhina, N.N.; Kostyuk, A.V.; Dzhabarov, E.G.; Filatova, M.P.; Antonov, S.V.; Maksimov, A.L. Hydrogenation of Indene–Coumarone Resin on Palladium Catalysts for Use in Polymer Adhesives. Russ. J. Appl. Chem. 2019, 92, 1143–1152. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Rheological and Adhesion Properties of Hot-Melt Adhesives Based on Hydrocarbon Resins and Poly(ethylene-vinyl acetate). Polym. Sci. Ser. A 2021, 63, 283–295. [Google Scholar] [CrossRef]
- Borodulina, T.; Bermesheva, E.; Smirnova, N.; Ilyin, S.; Brantseva, T.; Antonov, S. Adhesive properties of liquid crystalline hydroxypropyl cellulose–propylene glycol blends. J. Adhes. Sci. Technol. 2014, 28, 1629–1643. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kostyuk, A.V.; Ignatenko, V.Y.; Smirnova, N.M.; Alekseeva, O.A.; Petrukhina, N.N.; Antonov, S.V. The Effect of Tackifier on the Properties of Pressure-Sensitive Adhesives Based on Styrene–Butadiene–Styrene Rubber. Russ. J. Appl. Chem. 2018, 91, 1945–1956. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, D.Y.; Yoon, S.H.; Kim, S.H.; Han, M.S.; Jeon, S.; Kim, Y.; Lim, Y.K.; Hwang, D.-H.; Jung, S.-H.; et al. Adhesion Improvement of Solvent-Free Pressure-Sensitive Adhesives by Semi-IPN Using Polyurethanes and Acrylic Polymers. Polymers 2022, 14, 3963. [Google Scholar] [CrossRef]
- Ortega-Iguña, M.; Chludzinski, M.; Sánchez-Amaya, J.M. Comparative Mechanical Study of Pressure Sensitive Adhesives over Aluminium Substrates for Industrial Applications. Polymers 2022, 14, 4783. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Rokicka, J.; Klimowicz, A.; Czech, Z. Sustainable UV-Crosslinkable Acrylic Pressure-Sensitive Adhesives for Medical Application. Int. J. Mol. Sci. 2021, 22, 11840. [Google Scholar] [CrossRef]
- Gibert, F.; Allal, A.; Marin, G.; Derail, C. Effect of the rheological properties of industrial hot-melt and pressure-sensitive adhesives on the peel behavior. J. Adhes. Sci. Technol. 1999, 13, 1029–1044. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martin-Martínez, J.M. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2018, 88, 81–90. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The Effect of Type-I Photoinitiators on the Kinetics of the UV-Induced Cotelomerization Process of Acrylate Monomers and Properties of Obtained Pressure-Sensitive Adhesives. Materials 2021, 14, 4563. [Google Scholar] [CrossRef]
- Feldstein, M.M.; Dormidontova, E.E.; Khokhlov, A.R. Pressure sensitive adhesives based on interpolymer complexes. Prog. Polym. Sci. 2015, 42, 79–153. [Google Scholar] [CrossRef]
- O’Brien, E.P.; Germinario, L.T.; Robe, G.R.; Williams, T.; Atkins, D.G.; Moroney, D.A.; Peters, M.A. Fundamentals of hot-melt pressure-sensitive adhesive tapes: The effect of tackifier aromaticity. J. Adhes. Sci. Technol. 2007, 21, 637–661. [Google Scholar] [CrossRef]
- Benedek, I.; Feldstein, M. (Eds.) Handbook of Pressure-Sensitive Adhesives and Products: Technology of Pressure-Sensitive Adhesives and Products, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Mičušík, M.; Bonnefond, A.; Paulis, M.; Leiza, J.R. Synthesis of waterborne acrylic/clay nanocomposites by controlled surface initiation from macroinitiator modified montmorillonite. Eur. Polym. J. 2012, 48, 896–905. [Google Scholar] [CrossRef]
- Heck, C.A.; dos Santos, J.H.Z.; Wolf, C.R. Waterborne polyurethane: The effect of the addition or in situ formation of silica on mechanical properties and adhesion. Int. J. Adhes. Adhes. 2015, 58, 13–20. [Google Scholar] [CrossRef]
- Kajtna, J.; Šebenik, U. Microsphere pressure sensitive adhesives—acrylic polymer/montmorillonite clay nanocomposite materials. Int. J. Adhes. Adhes. 2009, 29, 543–550. [Google Scholar] [CrossRef]
- Brantseva, T.; Antonov, S.; Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Korolev, Y.; Tereshin, A.; Ilyin, S. Rheological and adhesive properties of PIB-based pressure-sensitive adhesives with montmorillonite-type nanofillers. Eur. Polym. J. 2016, 76, 228–244. [Google Scholar] [CrossRef]
- Pang, B.; Ryu, C.-M.; Kim, H.-I. Improvement of thermal stability of UV curable pressure sensitive adhesive by surface modified silica nanoparticles. Mater. Sci. Eng. B 2013, 178, 1212–1218. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and shear resistance. J. Adhes. Sci. Technol. 2014, 29, 1831–1848. [Google Scholar] [CrossRef]
- Songtipya, L.; Songtipya, P.; Sengsuk, T.; Kalkornsurapranee, E.; Nakaramontri, Y.; Johns, J. Improved adhesion properties of natural rubber-based pressure-sensitive adhesives by incorporating particulate fillers. Compos. Commun. 2021, 27, 100880. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Ignatenko, V.Y.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Polyethylene wax as an alternative to mineral fillers for preparation of reinforced pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2020, 102, 102689. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Two-functional phase-change pressure-sensitive adhesives based on polyisobutylene matrix filled with paraffin wax. J. Energy Storage 2022, 52, 104797. [Google Scholar] [CrossRef]
- Park, G.H.; Kim, K.T.; Ahn, Y.T.; Lee, H.-I.; Jeong, H.M. The effects of graphene on the properties of acrylic pressure-sensitive adhesive. J. Ind. Eng. Chem. 2014, 20, 4108–4111. [Google Scholar] [CrossRef]
- Kumar, S.K.; Krishnamoorti, R. Nanocomposites: Structure, Phase Behavior, and Properties. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.K.; Keum, J.; Boeman, R.G. Polymer matrix nanocomposites for automotive structural components. Nat. Nanotechnol. 2016, 11, 1026–1030. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Brantseva, T.V.; Kotomin, S.V.; Antonov, S.V. Epoxy nanocomposites as matrices for aramid fiber-reinforced plastics. Polym. Compos. 2017, 39, E2167–E2174. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, J.; Zuo, Y.; Li, H.; Cao, Y. Montmorillonite/polypropylene nanocomposites: Mechanical properties, crystallization and rheological behaviors. Appl. Clay Sci. 2011, 52, 171–178. [Google Scholar] [CrossRef]
- Antosik, A.K.; Mozelewska, K. Influence of Nanoclay on the Thermo-Mechanical Properties of Silicone Pressure-Sensitive Adhesives. Materials 2022, 15, 7460. [Google Scholar] [CrossRef]
- DeLozier, D.M.; Working, D.C. Polyetherimide/Montmorillonite Nanocomposites via in-situ Polymerization followed by Melt Processing. High Perform. Polym. 2004, 16, 597–609. [Google Scholar] [CrossRef]
- Brantseva, T.; Ilyin, S.; Gorbunova, I.; Antonov, S.; Korolev, Y.; Kerber, M. Epoxy reinforcement with silicate particles: Rheological and adhesive properties—Part II: Characterization of composites with halloysite. Int. J. Adhes. Adhes. 2016, 68, 248–255. [Google Scholar] [CrossRef]
- Akram, N.; Saeed, M.; Usman, M.; Mansha, A.; Anjum, F.; Zia, K.; Mahmood, I.; Mumtaz, N.; Khan, W.G. Influence of Graphene Oxide Contents on Mechanical Behavior of Polyurethane Composites Fabricated with Different Diisocyanates. Polymers 2021, 13, 444. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Hamid, Z.A. Graphene-Based Nanocomposites: Synthesis, Mechanical Properties, and Characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef]
- Das, T.K.; Prusty, S. Graphene-Based Polymer Composites and Their Applications. Polym. Technol. Eng. 2013, 52, 319–331. [Google Scholar] [CrossRef]
- Ilyin, S.; Arinina, M.; Polyakova, M.; Bondarenko, G.; Konstantinov, I.; Kulichikhin, V.; Malkin, A. Asphaltenes in heavy crude oil: Designation, precipitation, solutions, and effects on viscosity. J. Pet. Sci. Eng. 2016, 147, 211–217. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Pakhmanova, O.A.; Kostyuk, A.V.; Antonov, S.V. Effect of the Asphaltene, Resin, and Wax Contents on the Physicochemical Properties and Quality Parameters of Crude Oils. Pet. Chem. 2017, 57, 1141–1143. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Strelets, L.A. Basic Fundamentals of Petroleum Rheology and Their Application for the Investigation of Crude Oils of Different Natures. Energy Fuels 2018, 32, 268–278. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, rheology and possible application of water-in-oil emulsions stabilized by asphaltenes. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Kamkar, M.; Natale, G. A review on novel applications of asphaltenes: A valuable waste. Fuel 2020, 285, 119272. [Google Scholar] [CrossRef]
- Wu, H.; Kessler, M.R. Asphaltene: Structural characterization, molecular functionalization, and application as a low-cost filler in epoxy composites. RSC Adv. 2015, 5, 24264–24273. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Torres, S.W.; Messman, J.; Bowen, D.E.; Bowler, N. Dynamics of model polycyclic aromatic hydrocarbon compound-epoxy composites: A dielectric study. Polymer 2018, 136, 6–16. [Google Scholar] [CrossRef]
- Han, X.; Su, W.; Gong, J.; Xi, Z.; Zhang, J.; Cai, J.; Wang, Q.; Xie, H. Microstructure and dynamic mechanical properties epoxy/asphaltene composites. J. Therm. Anal. Calorim. 2021, 147, 2209–2219. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Kostina, J.V.; Bakhtin, D.S.; Makarova, V.V.; Antonov, S.V.; Ilyin, S.O. Heavy crude oil asphaltenes as a nanofiller for epoxy resin. Polym. Eng. Sci. 2020, 60, 1530–1545. [Google Scholar] [CrossRef]
- Miao, W.; Zhu, H.; Duan, T.; Chen, H.; Wu, F.; Jiang, L.; Wang, Z.; Weijun, M.; Hao, Z.; Hongbing, C.; et al. High-density polyethylene crystals with double melting peaks induced by ultra-high-molecular-weight polyethylene fibre. R. Soc. Open Sci. 2018, 5, 180394. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Alghizzi, A.G.; Suliman, M.H.; Achilias, D.S. Effect of Natural Macromolecule Filler on the Properties of High-Density Polyethylene (HDPE). Macromol. Symp. 2018, 380, 1800072. [Google Scholar] [CrossRef]
- Minzagirova, A.M.; Gilmanova, A.R.; Borisova, Y.Y.; Borisov, D.N.; Galikhanov, M.F.; Ziganshin, M.A.; Yakubov, M.R. Polyolefin Composition Materials Filled with Oil Asphaltenes and their Functionalized Derivatives. J. Sib. Fed. Univ. Chem. 2020, 13, 408–417. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Hussain, S.; Achilias, D.S. Use of asphaltene filler to improve low-density polyethylene properties. Pet. Sci. Technol. 2018, 36, 756–764. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Preparation and properties of polypropylene-asphaltene composites. Polym. Compos. 2015, 38, 1957–1963. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Using asphaltenes as filler in methyl methacrylate polymer composites. Pet. Sci. Technol. 2016, 34, 253–259. [Google Scholar] [CrossRef]
- Eshraghian, A.; Kamkar, M.; Sundararaj, U. Asphaltene/polymer composites: Morphology, compatibility, and rheological properties. Can. J. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Siddiqui, M.N. Studies of Different Properties of Polystyrene-Asphaltene Composites. Macromol. Symp. 2015, 354, 184–190. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Antonov, S.V.; Kostyuk, A.V.; Smirnova, N.M.; Makarova, V.V.; Ilyin, S.O. Composites Based on Polystyrene and Asphaltenes. Russ. J. Appl. Chem. 2019, 92, 1712–1717. [Google Scholar] [CrossRef]
- Wu, H.; Thakur, V.K.; Kessler, M.R. Novel low-cost hybrid composites from asphaltene/SBS tri-block copolymer with improved thermal and mechanical properties. J. Mater. Sci. 2015, 51, 2394–2403. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Asphaltenes as a tackifier for hot-melt adhesives based on the styrene-isoprene-styrene block copolymer. Polym. Eng. Sci. 2020, 60, 2224–2234. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M. Hot-Melt and Pressure-Sensitive Adhesives Based on Styrene-Isoprene-Styrene Triblock Copolymer, Asphaltene/Resin Blend and Naphthenic Oil. Polymers 2022, 14, 4296. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, I.N.; Fesan, A.A.; Losev, A.P. Asphaltenes: Absorbers and Scatterers at Near-Ultraviolet–Visible–Near-Infrared Wavelengths. Energy Fuels 2017, 31, 3878–3884. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Younas, M.; Al-Arfaj, A.A.; Hussain, S.; Naim, M. Durability study of asphaltene-reinforced HDPE and LDPE composites under UV irradiation and local weathering exposure. Polym. Bull. 2020, 78, 4487–4503. [Google Scholar] [CrossRef]
- Zosel, A. Shear Strength of Pressure Sensitive Adhesives and its Correlation to Mechanical Properties. J. Adhes. 1994, 44, 1–16. [Google Scholar] [CrossRef]
- Krenceski, M.A.; Johnson, J.F. Shear, tack, and peel of polyisobutylene: Effect of molecular weight and molecular weight distribution. Polym. Eng. Sci. 1989, 29, 36–43. [Google Scholar] [CrossRef]
- Ilyin, S.; Arinina, M.; Polyakova, M.; Kulichikhin, V.; Malkin, A. Rheological comparison of light and heavy crude oils. Fuel 2016, 186, 157–167. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Compatibility and rheology of bio-oil blends with light and heavy crude oils. Fuel 2021, 314, 122761. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Kostina, Y.V.; Antonov, S.V.; Ilyin, S.O. Oxidative Functionalization of Asphaltenes from Heavy Crude Oil. Russ. J. Appl. Chem. 2018, 91, 1835–1840. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Ignatenko, V.Y.; Kostyuk, A.V.; Levin, I.S.; Bondarenko, G.N. Deasphalting of heavy crude oil by hexamethyldisiloxane: The effect of a solvent/oil ratio on the structure, composition, and properties of precipitated asphaltenes. J. Pet. Sci. Eng. 2021, 208, 109329. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of shear rate and viscosity from batch mixer data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G. Application of large amplitude oscillatory shear for the analysis of polymer material properties in the nonlinear mechanical behavior. Polym. Sci. Ser. A 2014, 56, 98–110. [Google Scholar] [CrossRef]
- Kroger, M. Characterization of material viscoelasticity at large deformations. Appl. Rheol. 2014, 24, 9–18. [Google Scholar] [CrossRef]
- Strelets, L.A.; Ilyin, S.O. Effect of enhanced oil recovery on the composition and rheological properties of heavy crude oil. J. Pet. Sci. Eng. 2021, 203, 108641. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and adhesive properties of nanocomposite bitumen binders based on hydrophilic or hydrophobic silica and modified with bio-oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological properties of polyethylene/metaboric acid thermoplastic blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Palierne, J.F. Linear rheology of viscoelastic emulsions with interfacial tension. Rheol. Acta 1990, 29, 204–214. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E. Interfacial viscoelasticity in emulsions and foams. Food Struct. 1993, 12, 1–12. [Google Scholar]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase state and rheology of polyisobutylene blends with silicone resin. Rheol. Acta 2020, 59, 375–386. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Bitumen improvement with bio-oil and natural or organomodified montmorillonite: Structure, rheology, and adhesion of composite asphalt binders. Constr. Build. Mater. 2023, 364, 129919. [Google Scholar] [CrossRef]
- Nifant’Ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sadrtdinova, G.I.; Komarov, P.D.; Minyaev, M.E.; Ilyin, S.O.; Kiselev, A.V.; Samurganova, T.I.; Ivchenko, P.V. Synthesis, molecular structure and catalytic performance of heterocycle-fused cyclopentadienyl-amido CGC of Ti (IV) in ethylene (co)polymerization: The formation and precision rheometry of long-chain branched polyethylenes. Eur. Polym. J. 2022, 176, 111397. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The rheological state of suspensions in varying the surface area of nano-silica particles and molecular weight of the poly(ethylene oxide) matrix. Colloid Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic nanosilica-stabilized graphite particles for improving thermal conductivity of paraffin wax-based phase-change materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Rehbinder, P.A. Formation of structures in disperse systems. Pure Appl. Chem. 1965, 10, 337–358. [Google Scholar] [CrossRef]
- Sengsuk, T.; Songtipya, P.; Kalkornsurapranee, E.; Johns, J.; Songtipya, L. Active Bio-Based Pressure-Sensitive Adhesive Based Natural Rubber for Food Antimicrobial Applications: Effect of Processing Parameters on Its Adhesion Properties. Polymers 2021, 13, 199. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekhina, V.Y.; Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding. Materials 2023, 16, 1209. https://doi.org/10.3390/ma16031209
Melekhina VY, Kostyuk AV, Smirnova NM, Ilyin SO. Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding. Materials. 2023; 16(3):1209. https://doi.org/10.3390/ma16031209
Chicago/Turabian StyleMelekhina, Viktoria Y., Anna V. Kostyuk, Nina M. Smirnova, and Sergey O. Ilyin. 2023. "Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding" Materials 16, no. 3: 1209. https://doi.org/10.3390/ma16031209
APA StyleMelekhina, V. Y., Kostyuk, A. V., Smirnova, N. M., & Ilyin, S. O. (2023). Asphaltene-Stabilized Polyisobutylene Pressure-Sensitive Adhesives for Ultraviolet Protection and Surface Bonding. Materials, 16(3), 1209. https://doi.org/10.3390/ma16031209




.png)