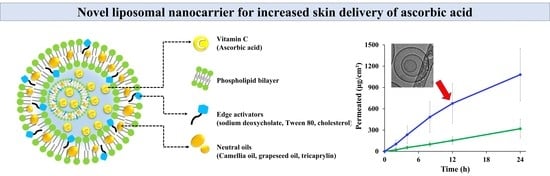
Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Vit C-Loaded LOS Systems
2.3. Physicochemical Characterization of LOS Formulations
2.3.1. Morphology of Liposomal Formulations
2.3.2. Liposomal Vesicle Size and Zeta Potential
2.3.3. Vit C Content Analysis
2.3.4. Loading Efficiency and Amount of Vit C in LOS Systems
2.4. Ex Vivo Skin Absorption of Vit C-Loaded Lipo-Oil-Some (LOS) Formulations
2.5. Statistical Analysis
3. Results and Discussion
3.1. Formulation Strategy and Characterization of LOS Systems with Different Edge Activators
3.2. Ex Vivo Skin Absorption Profiles of LOS Systems with Different Edge Activators
3.3. Characterization of LOS Systems with Different Neutral Oils
3.4. Ex Vivo Skin Absorption Profiles of LOS Systems with Different Neutral Oils
3.5. Characterization of LOS Systems with Different Amounts of Tric
3.6. Ex Vivo Skin Absorption Profiles of LOS Systems with Different Amounts of Tric
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telang, P.S. Vitamin C in Dermatology. Indian Derm. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef]
- Enescu, C.D.; Bedford, L.M.; Potts, G.; Fahs, F. A Review of Topical Vitamin C Derivatives and Their Efficacy. J. Cosmet. Dermatol. 2022, 21, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Tai, A.; Fujinami, Y.; Sasaki, K.; Okazaki, S. Synthesis and Characterization of a Series of Novel Monoacylated Ascorbic Acid Derivatives, 6-O-Acyl-2-O-Alpha-D-Glucopyranosyl-L-Ascorbic Acids, as Skin Antioxidants. J. Med. Chem. 2002, 45, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Horino, Y.; Takahashi, S.; Miura, T.; Takahashi, Y. Prolonged Hypoxia Accelerates the Posttranscriptional Process of Collagen Synthesis in Cultured Fibroblasts. Life Sci. 2002, 71, 3031–3045. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Hu, C.; Ma, C.; Fang, Q.; Xing, T.; Xia, Q. Development and Characterization of Solid Lipid Microparticles Containing Vitamin C for Topical and Cosmetic Use. Eur. J. Lipid Sci. Technol. 2016, 118, 1093–1103. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal Delivery of Molecules Is Limited by Full Epidermis, Not Just Stratum Corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Barra, P.A.; Márquez, K.; Gil-Castell, O.; Mujica, J.; Ribes-Greus, A.; Faccini, M. Spray-Drying Performance and Thermal Stability of L-Ascorbic Acid Microencapsulated with Sodium Alginate and Gum Arabic. Molecules 2019, 24, 2872. [Google Scholar] [CrossRef] [Green Version]
- Farias, M.D.P.; Albuquerque, P.B.S.; Soares, P.A.G.; de Sá, D.M.A.T.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Xyloglucan from Hymenaea Courbaril Var. Courbaril Seeds as Encapsulating Agent of l-Ascorbic Acid. Int. J. Biol. Macromol. 2018, 107, 1559–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comunian, T.A.; Thomazini, M.; Alves, A.J.G.; de Matos Junior, F.E.; de Carvalho Balieiro, J.C.; Favaro-Trindade, C.S. Microencapsulation of Ascorbic Acid by Complex Coacervation: Protection and Controlled Release. Food Res. Int. 2013, 52, 373–379. [Google Scholar] [CrossRef]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic Acid Encapsulated into Negatively Charged Liposomes Exhibits Increased Skin Permeation, Retention and Enhances Collagen Synthesis by Fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Wu, Z.; Qi, X. High Skin Permeation, Deposition and Whitening Activity Achieved by Xanthan Gum String Vitamin c Flexible Liposomes for External Application. Int. J. Pharm. 2022, 628, 122290. [Google Scholar] [CrossRef]
- Wölk, C.; Nawaz, H.A.; Maqsood, I.; Strati, F.; Brezesinski, G.; Hause, G.; Schulz-Siegmund, M.; Hacker, M.C. Amphiphilic Functionalized Oligomers: A Promising Strategy for the Postfabrication Functionalization of Liposomes. Adv. Mater. Interfaces 2020, 7, 2001168. [Google Scholar] [CrossRef]
- Kim, J.-S. Liposomal Drug Delivery System. J. Pharm. Investig. 2016, 46, 387–392. [Google Scholar] [CrossRef]
- Schätzlein, A.; Cevc, G. Non-Uniform Cellular Packing of the Stratum Corneum and Permeability Barrier Function of Intact Skin: A High-Resolution Confocal Laser Scanning Microscopy Study Using Highly Deformable Vesicles (Transfersomes). Br. J. Dermatol. 1998, 138, 583–592. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle Size of Liposomes Influences Dermal Delivery of Substances into Skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Sen, A.; Zhao, Y.; Zhang, L.; Hui, S.W. Enhanced Transdermal Transport by Electroporation Using Anionic Lipids. J. Control. Release 2002, 82, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.J.; Shafaat, K.; Faruk, A. Elastic Liposomes as Novel Carriers: Recent Advances in Drug Delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielińska, A.; Matos, C.M. Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef] [PubMed]
- Mack Correa, M.C.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular Interactions of Plant Oil Components with Stratum Corneum Lipids Correlate with Clinical Measures of Skin Barrier Function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef]
- Naik, A.; Pechtold, L.A.R.M.; Potts, R.O.; Guy, R.H. Mechanism of Oleic Acid-Induced Skin Penetration Enhancement in Vivo in Humans. J. Control. Release 1995, 37, 299–306. [Google Scholar] [CrossRef]
- Ho, M.J.; Park, D.W.; Kang, M.J. Design of Novel Tricaprylin-Incorporated Multi-Layered Liposomal System for Skin Delivery of Ascorbic Acid with Improved Chemical Stability. Pharmaceuticals 2023, 16, 121. [Google Scholar] [CrossRef]
- Kaul, S.; Jain, N.; Nagaich, U. Ultra Deformable Vesicles for Boosting Transdermal Delivery of 2-Arylpropionic Acid Class Drug for Management of Musculoskeletal Pain. J. Pharm. Investig. 2022, 52, 217–231. [Google Scholar] [CrossRef]
- Rehan, F.; Emranul Karim, M.; Ahemad, N.; Farooq Shaikh, M.; Gupta, M.; Gan, S.H.; Chowdhury, E.H. A Comparative Evaluation of Anti-Tumor Activity Following Oral and Intravenous Delivery of Doxorubicin in a Xenograft Model of Breast Tumor. J. Pharm. Investig. 2022, 52, 787–804. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Liew, Q.X.; Ng, K.Y.; Chowdhury, E.H. Active Targeting via Ligand-Anchored PH-Responsive Strontium Nanoparticles for Efficient Nucleic Acid Delivery into Breast Cancer Cells. J. Pharm. Investig. 2022, 52, 243–257. [Google Scholar] [CrossRef]
- Gutiérrez-Quequezana, L.; Vuorinen, A.L.; Kallio, H.; Yang, B. Impact of Cultivar, Growth Temperature and Developmental Stage on Phenolic Compounds and Ascorbic Acid in Purple and Yellow Potato Tubers. Food Chem. 2020, 326, 126966. [Google Scholar] [CrossRef] [PubMed]
- Lertpairod, J.; Tiyaboonchai, W. pH-Sensitive Beads Containing Curcumin Loaded Nanostructured Lipid Carriers for a Colon Targeted Oral Delivery System. J. Pharm. Investig. 2022, 52, 387–396. [Google Scholar] [CrossRef]
- Neri, I.; Laneri, S.; Di Lorenzo, R.; Dini, I.; Russo, G.; Grumetto, L. Parabens Permeation through Biological Membranes: A Comparative Study Using Franz Cell Diffusion System and Biomimetic Liquid Chromatography. Molecules 2022, 27, 4263. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol Injection Method for Hydrophilic and Lipophilic Drug-Loaded Liposome Preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Loureiro, A.; Lager, F.; Renault, G.; Cavaco-Paulo, A.; Nogueira, E. Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomedicines 2020, 8, 630. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of Edge Activators and Surface Charge in Developing Ultradeformable Vesicles with Enhanced Skin Delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of Carvedilol for Intranasal Delivery: Formulation, Characterization and in Vivo Evaluation. Drug Deliv. 2016, 23, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiyana, W.; Leelapornpisid, P.; Jakmunee, J.; Korsamphan, C. Antioxidant and Moisturizing Effect of Camellia Assamica Seed Oil and Its Development into Microemulsion. Cosmetics 2018, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, R.; Gagliardi, A.; Mancuso, A.; Cosco, D.; Soliman, M.E.; Casettari, L.; Paolino, D. Development and In Vivo Evaluation of Multidrug Ultradeformable Vesicles for the Treatment of Skin Inflammation. Pharmaceutics 2019, 11, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine Ear Skin: An in Vitro Model for Human Skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Gray, G.M.; Yardley, H.J. Lipid Compositions of Cells Isolated from Pig, Human, and Rat Epidermis. J. Lipid Res. 1975, 16, 434–440. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin Models for the Testing of Transdermal Drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Fornasier, F.; Souza, L.M.P.; Souza, F.R.; Reynaud, F.; Pimentel, A.S. Lipophilicity of Coarse-Grained Cholesterol Models. J. Chem. Inf. Model. 2020, 60, 569–577. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Sharma, U.; Verma, P.; Jain, N.K. A Review on Novel Vesicular Drug Delivery System: Transfersomes. Int. J. Pharm. Life Sci. 2020, 11, 6812–6824. [Google Scholar]
- Opatha, S.A.T.; Titapiwatanakun, V.; Boonpisutiinant, K.; Chutoprapat, R. Preparation, Characterization and Permeation Study of Topical Gel Loaded with Transfersomes Containing Asiatic Acid. Molecules 2022, 27, 4865. [Google Scholar] [CrossRef] [PubMed]
- Almehmady, A.M.; Elsisi, A.M. Development, Optimization, and Evaluation of Tamsulosin Nanotransfersomes to Enhance Its Permeation and Bioavailability. J. Drug Deliv. Sci. Technol. 2020, 57, 101667. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Zhang, X.; Huang, J.; Yu, X.; Li, J.; Xiong, D.; Sun, X.; Zhong, Z. Effect of a Controlled-Release Drug Delivery System Made of Oleanolic Acid Formulated into Multivesicular Liposomes on Hepatocellular Carcinoma in Vitro and in Vivo. Int. J. Nanomed. 2016, 11, 3111–3129. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Kobarfard, F.; Javed, Z.; Rajabi, S.; Khan, K.; Ashfaq, H.A.; Ahmad, T.; Pezzani, R.; et al. Multivesicular Liposome (Depofoam) in Human Diseases. Iran J. Pharm. Res. 2020, 19, 9–21. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, H.G.; Kim, C.R.; Lim, H.-J.; Cho, K.M.; Choi, J.S.; Shin, D.-H.; Shin, E.-C. Quality Evaluation on Use of Camellia Oil as an Alternative Method in Dried Seaweed Preparation. Prev. Nutr. Food Sci. 2014, 19, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J.G. Membrane Composition and Dynamics: A Target of Bioactive Virgin Olive Oil Constituents. Biochim. Biophys. Acta-Biomembra. 2014, 1838, 1638–1656. [Google Scholar] [CrossRef] [Green Version]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. Exploring the Potential of Oleic Acid in Nanotechnology-Mediated Dermal Drug Delivery: An up-to-Date Review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Harayama, T.; Shimizu, T. Roles of Polyunsaturated Fatty Acids, from Mediators to Membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liang, R.; Liu, C.; Chen, M.; Chen, J. Synergistic Anti-Inflammatory Effects of Lipophilic Grape Seed Proanthocyanidin and Camellia Oil Combination in LPS-Stimulated RAW264.7 Cells. Antioxidants 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Mosquera Narvaez, L.E.; Ferreira, L.M.d.M.C.; Sanches, S.; Alesa Gyles, D.; Silva-Júnior, J.O.C.; Ribeiro Costa, R.M. A Review of Potential Use of Amazonian Oils in the Synthesis of Organogels for Cosmetic Application. Molecules 2022, 27, 2733. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Seki, T.; Yuan, D.; Saso, Y.; Hosoya, O.; Chono, S.; Morimoto, K. Effect of Camellia Oil on the Permeation of Flurbiprofen and Diclofenac Sodium through Rat and Pig Skin. Biol. Pharm. Bull. 2004, 27, 1476–1479. [Google Scholar] [CrossRef] [Green Version]
- Francoeur, M.L.; Golden, G.M.; Potts, R.O. Oleic Acid: Its Effects on Stratum Corneum in Relation to (Trans)Dermal Drug Delivery. Pharm. Res. 1990, 7, 621–627. [Google Scholar] [CrossRef]
- Srisuk, P.; Thongnopnua, P.; Raktanonchai, U.; Kanokpanont, S. Physico-Chemical Characteristics of Methotrexate-Entrapped Oleic Acid-Containing Deformable Liposomes for in Vitro Transepidermal Delivery Targeting Psoriasis Treatment. Int. J. Pharm. 2012, 427, 426–434. [Google Scholar] [CrossRef] [PubMed]






| Formulation No. | L1 | L2 | L3 | L4 | L5 | L6 | L7 |
|---|---|---|---|---|---|---|---|
| Vitamin C (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Phosphatidylcholine (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| DPPG (mg) | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Cholesterol (mg) | 20 | - | - | - | - | - | - |
| Sodium deoxycholate (mg) | - | 20 | - | 20 | 20 | 20 | 20 |
| Polysorbate 80 (mg) | - | - | 20 | - | - | - | - |
| Camellia oil (mg) | 200 | 200 | 200 | - | - | - | - |
| Grapeseed oil (mg) | - | - | - | 200 | - | - | - |
| Tricaprylin (mg) | - | - | - | - | 200 | 500 | 800 |
| 10 mM succinate buffer | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Total (mL) | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| pH (a) | 3.5 | 3.2 | 3.4 | 3.2 | 3.3 | 3.2 | 3.2 |
| Chol (L1) | Sod.DC (L2) | T80 (L3) | |
|---|---|---|---|
| Flux (μg/cm2∙h) | 13.0 ± 6.07 | 45.4 ± 15.3 * | 38.7 ± 6.09 * |
| Lag time (h) | 0.40 ± 0.07 †,** | 1.28 ± 0.63 | 1.32 ± 0.61 |
| Permeability coefficient (10−6∙cm/h) | 0.65 ± 0.21 | 2.27 ± 0.54 * | 1.93 ± 0.23 * |
| Permeated (μg/cm2) | 318.8 ± 131.8 | 1080.3 ± 370.3 * | 845.2 ± 193.5 * |
| CO (L2) | GO (L4) | Tric (L5) | |
|---|---|---|---|
| Flux (μg/cm2∙h) | 45.4 ± 15.3 | 25.3 ± 6.85 | 35.5 ± 9.29 |
| Lag time (h) | 1.28 ± 0.63 | 1.08 ± 0.38 | 2.27 ± 1.10 |
| Permeability coefficient (10−6∙cm/h) | 2.27 ± 0.54 † | 1.26 ± 0.23 | 1.78 ± 0.34 |
| Permeated (μg/cm2) | 1080.3 ± 370.31 | 592.2 ± 182.6 | 868.0 ± 242.3 |
| Tric 2% (L5) | Tric 5% (L6) | Tric 8% (L7) | |
|---|---|---|---|
| Flux (μg/cm2∙h) | 35.5 ± 9.29 | 22.6 ± 4.00 | 23.9 ± 10.3 |
| Lag time (h) | 2.27 ± 1.10 | 0.12 ± 0.01 * | 3.09 ± 4.38 |
| Permeability coefficient (10−6∙cm/h) | 1.78 ± 0.34 † | 1.13 ± 0.14 | 1.20 ± 0.39 |
| Permeated (μg/cm2) | 868.0 ± 242.3 † | 524.3 ± 100.2 | 585.2 ± 218.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, M.J.; Park, H.J.; Kang, M.J. Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid. Materials 2023, 16, 2294. https://doi.org/10.3390/ma16062294
Ho MJ, Park HJ, Kang MJ. Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid. Materials. 2023; 16(6):2294. https://doi.org/10.3390/ma16062294
Chicago/Turabian StyleHo, Myoung Jin, Hyun Jin Park, and Myung Joo Kang. 2023. "Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid" Materials 16, no. 6: 2294. https://doi.org/10.3390/ma16062294
APA StyleHo, M. J., Park, H. J., & Kang, M. J. (2023). Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid. Materials, 16(6), 2294. https://doi.org/10.3390/ma16062294