CALPHAD-Based Modelling of the Temperature–Composition–Structure Relationship during Physical Vapor Deposition of Mg-Ca Thin Films
Abstract
:1. Introduction
2. Materials and Methods
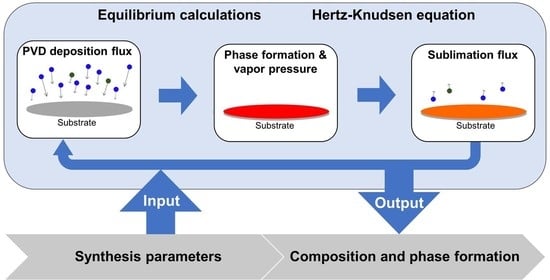
2.1. Theoretical Methods
2.2. Experimental Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Löffler, F.H. Formation of Non-Equilibrium Phases in the PVD Process. Vacuum 1992, 43, 397–402. [Google Scholar] [CrossRef]
- Spencer, P. Thermodynamic Prediction of Metastable Coating Structures in PVD Processes. Int. J. Mater. Res. 2001, 92, 1145–1150. [Google Scholar] [CrossRef]
- Chang, K.; Music, D.; to Baben, M.; Lange, D.; Bolvardi, H.; Schneider, J.M. Modeling of Metastable Phase Formation Diagrams for Sputtered Thin Films. Sci. Technol. Adv. Mater. 2016, 17, 210–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; to Baben, M.; Music, D.; Lange, D.; Bolvardi, H.; Schneider, J.M. Estimation of the Activation Energy for Surface Diffusion during Metastable Phase Formation. Acta Mater. 2015, 98, 135–140. [Google Scholar] [CrossRef]
- Liu, S.; Chang, K.; Mráz, S.; Chen, X.; Hans, M.; Music, D.; Primetzhofer, D.; Schneider, J.M. Modeling of Metastable Phase Formation for Sputtered Ti1-XAlxN Thin Films. Acta Mater. 2019, 165, 615–625. [Google Scholar] [CrossRef]
- to Baben, M.; Hans, M.; Primetzhofer, D.; Evertz, S.; Ruess, H.; Schneider, J.M. Unprecedented Thermal Stability of Inherently Metastable Titanium Aluminum Nitride by Point Defect Engineering. Mater. Res. Lett. 2017, 5, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Bai, Y.; Qu, W.; Wu, A.; Zhang, D.; Zhao, J.; Jiang, X. Al–Mg–B Thin Films Prepared by Magnetron Sputtering. Vacuum 2010, 85, 541–545. [Google Scholar] [CrossRef]
- Beckers, M.; Höglund, C.; Baehtz, C.; Martins, R.M.S.; Persson, P.O.Å.; Hultman, L.; Möller, W. The Influence of Substrate Temperature and Al Mobility on the Microstructural Evolution of Magnetron Sputtered Ternary Ti–Al–N Thin Films. J. Appl. Phys. 2009, 106, 064915. [Google Scholar] [CrossRef]
- Herrig, F. Ab Initio Guided Design of Thin Film Model Systems for FeMn Based Steels. Ph.D. Thesis, Rheinisch-Westfälische Technische Hochschule Aachen, Aachen, Germany, 2019. [Google Scholar]
- Högberg, H.; Emmerlich, J.; Eklund, P.; Wilhelmsson, O.; Palmquist, J.P.; Jansson, U.; Hultman, L. Growth and Property Characterization of Epitaxial MAX-Phase Thin Films from the Tin+1(Si, Ge, Sn)Cn Systems. Adv. Sci. Technol. 2006, 45, 2648–2655. [Google Scholar] [CrossRef]
- Wilhelmsson, O.; Eklund, P.; Högberg, H.; Hultman, L.; Jansson, U. Structural, Electrical and Mechanical Characterization of Magnetron-Sputtered V–Ge–C Thin Films. Acta Mater. 2008, 56, 2563–2569. [Google Scholar] [CrossRef]
- Keuter, P.; Karimi Aghda, S.; Music, D.; Kümmerl, P.; Schneider, J.M. Synthesis of Intermetallic (Mg1−x, Alx)2Ca by Combinatorial Sputtering. Materials 2019, 12, 3026. [Google Scholar] [CrossRef] [Green Version]
- Ham, B.; Junkew, A.; Bufford, D.; Arróyave, R.; Zhang, X. Fabrication of Porous and Pillar-Shaped Mg by Magnetron Sputtering. Thin Solid Film. 2014, 550, 220–226. [Google Scholar] [CrossRef]
- Structure and Corrosion of Magnetron Sputtered Pure Mg Films on Silicon Substrates—Störmer. 2007. Plasma Processes and Polymers. Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ppap.200731405 (accessed on 21 September 2022).
- Grigucevičienė, A.; Leinartas, K.; Juškėnas, R.; Juzeliūnas, E. Structure and Initial Corrosion Resistance of Sputter Deposited Nanocrystalline Mg–Al–Zr Alloys. Mater. Sci. Eng. A 2005, 394, 411–416. [Google Scholar] [CrossRef]
- Bouaziz, O.; Billard, A. Structure–Mechanical Properties Relationships of Co-Sputter Deposited Iron–Magnesium Coatings. Surf. Coat. Technol. 2007, 201, 7007–7012. [Google Scholar] [CrossRef]
- Ludwig, A.; Cao, J.; Dam, B.; Gremaud, R. Opto-Mechanical Characterization of Hydrogen Storage Properties of Mg–Ni Thin Film Composition Spreads. Appl. Surf. Sci. 2007, 254, 682–686. [Google Scholar] [CrossRef]
- Schmuelling, G.; Winter, M.; Placke, T. Investigating the Mg–Si Binary System via Combinatorial Sputter Deposition as High Energy Density Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 20124–20133. [Google Scholar] [CrossRef]
- Olk, C.H.; Haddad, D.B. Growth and Structure of a Combinatorial Array of Mixed-Phase Magnesium–Aluminum Thin-Film Alloys. Appl. Phys. A 2007, 88, 249–253. [Google Scholar] [CrossRef]
- Shedden, B.A.; Samandi, M.; Window, B. Stoichiometry of Unbalanced Magnetron Sputtered Al–Mg Alloy Coatings. Surf. Coat. Technol. 1997, 97, 557–563. [Google Scholar] [CrossRef]
- Yamada, Y.; Bao, S.; Tajima, K.; Okada, M.; Yoshimura, K. Optical Properties of Switchable Mirrors Based on Magnesium-Calcium Alloy Thin Films. Appl. Phys. Lett. 2009, 94, 191910. [Google Scholar] [CrossRef]
- Hans, M.; Keuter, P.; Saksena, A.; Sälker, J.A.; Momma, M.; Springer, H.; Nowak, J.; Zander, D.; Primetzhofer, D.; Schneider, J.M. Opportunities of Combinatorial Thin Film Materials Design for the Sustainable Development of Magnesium-Based Alloys. Sci. Rep. 2021, 11, 17454. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Ben Mahfoud, R.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage Thermochemical Software and Databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Jung, I.-H.; Zhu, Z.; Kim, J.; Wang, J.; Chartrand, P.; Pelton, A. Recent Progress on the Factsage Thermodynamic Database for New Mg Alloy Development. JOM 2017, 69, 1052–1059. [Google Scholar] [CrossRef]
- Safarian, J.; Engh, T.A. Vacuum Evaporation of Pure Metals. Metall. Mater. Trans. A 2013, 44, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Hołyst, R.; Litniewski, M.; Jakubczyk, D. A Molecular Dynamics Test of the Hertz–Knudsen Equation for Evaporating Liquids. Soft Matter 2015, 11, 7201–7206. [Google Scholar] [CrossRef] [PubMed]
- Aliramaji, S.; Keuter, P.; Neuß, D.; Hans, M.; Primetzhofer, D.; Depla, D.; Schneider, J.M. Effect of Growth Temperature and Atmosphere Exposure Time on Impurity Incorporation in Sputtered Mg, Al, and Ca Thin Films. Materials 2023, 16, 414. [Google Scholar] [CrossRef]
- Shao, L.; Shi, T.-T.; Zheng, J.; Pan, X.-Z.; Tang, B.-Y. The Native Point Defects in C14 Mg2Ca Laves Phase: A First-Principles Study. Intermetallics 2015, 65, 29–34. [Google Scholar] [CrossRef]
- Kevorkov, D.; Medraj, M.; Li, J.; Essadiqi, E.; Chartrand, P. The 400 °C Isothermal Section of the Mg–Al–Ca System. Intermetallics 2010, 18, 1498–1506. [Google Scholar] [CrossRef]
- Freund, M.; Andre, D.; Zehnder, C.; Rempel, H.; Gerber, D.; Zubair, M.; Sandlöbes-Haut, S.; Gibson, J.S.K.-L.; Korte-Kerzel, S. Plastic Deformation of the CaMg2 C14-Laves Phase from 50–250 °C. Materialia 2021, 20, 101237. [Google Scholar] [CrossRef]
- Suzuki, A.; Saddock, N.D.; Jones, J.W.; Pollock, T.M. Phase Equilibria in the Mg-Al-Ca Ternary System at 773 and 673 K. Metall. Mater. Trans. A 2006, 37, 975–983. [Google Scholar] [CrossRef]
- Amerioun, S.; Simak, S.I.; Häussermann, U. Laves-Phase Structural Changes in the System CaAl2-XMgx. Inorg. Chem. 2003, 42, 1467–1474. [Google Scholar] [CrossRef]
- Kozlov, A.; Ohno, M.; Arroyave, R.; Liu, Z.K.; Schmid-Fetzer, R. Phase Equilibria, Thermodynamics and Solidification Microstructures of Mg–Sn–Ca Alloys, Part 1: Experimental Investigation and Thermodynamic Modeling of the Ternary Mg–Sn–Ca System. Intermetallics 2008, 16, 299–315. [Google Scholar] [CrossRef]
- Xie, Z.; Chauraud, D.; Bitzek, E.; Korte-Kerzel, S.; Guénolé, J. Laves Phase Crystal Analysis (LaCA): Atomistic Identification of Lattice Defects in C14 and C15 Topologically Close-Packed Phases. J. Mater. Res. 2021, 36, 2010–2024. [Google Scholar] [CrossRef]
- Pinard, P.T.; Protheroe, A.; Holland, J.; Burgess, S.; Statham, P.J. Development and Validation of Standardless and Standards-Based X-ray Microanalysis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 891, 012020. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keuter, P.; to Baben, M.; Aliramaji, S.; Schneider, J.M. CALPHAD-Based Modelling of the Temperature–Composition–Structure Relationship during Physical Vapor Deposition of Mg-Ca Thin Films. Materials 2023, 16, 2417. https://doi.org/10.3390/ma16062417
Keuter P, to Baben M, Aliramaji S, Schneider JM. CALPHAD-Based Modelling of the Temperature–Composition–Structure Relationship during Physical Vapor Deposition of Mg-Ca Thin Films. Materials. 2023; 16(6):2417. https://doi.org/10.3390/ma16062417
Chicago/Turabian StyleKeuter, Philipp, Moritz to Baben, Shamsa Aliramaji, and Jochen M. Schneider. 2023. "CALPHAD-Based Modelling of the Temperature–Composition–Structure Relationship during Physical Vapor Deposition of Mg-Ca Thin Films" Materials 16, no. 6: 2417. https://doi.org/10.3390/ma16062417
APA StyleKeuter, P., to Baben, M., Aliramaji, S., & Schneider, J. M. (2023). CALPHAD-Based Modelling of the Temperature–Composition–Structure Relationship during Physical Vapor Deposition of Mg-Ca Thin Films. Materials, 16(6), 2417. https://doi.org/10.3390/ma16062417