Control of Magnetic Properties of NiMn2O4 by a Microwave Magnetic Field under Air
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Single-Phase NiMnO
2.2. Microwave Irradiation of NiMnO at the Maximum Point of Microwave H-Field Intensity
3. Results and Discussion
3.1. Phase and Microstructure after Microwave H-Field Irradiation
3.2. Magnetic Properties of H-Field-Irradiated NiMnO
3.3. Effect of Annealing after Microwave Irradiation
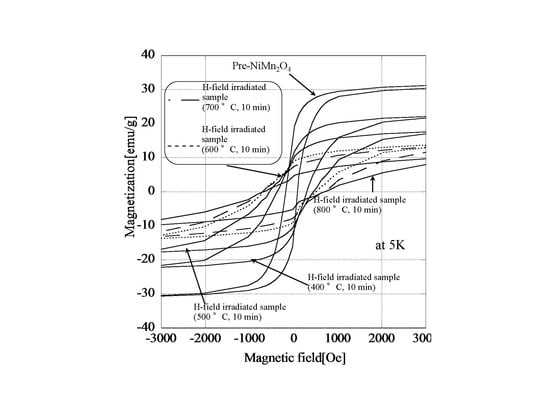
3.4. Controlling the Magnetic Properties of NiMnO by a Microwave H-Field at Different Temperatures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsujimoto, Y.; Tassel, C.; Hayashi, N.; Watanabe, T.; Kageyama, H.; Yoshimura, K.; Takano, M.; Ceretti, M.; Ritter, C.; Paulus, W. Infinite-layer iron oxide with a square-planar coordination. Nature 2007, 450, 1062–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, H.; Watanabe, T.; Tsujimoto, Y.; Kitada, A.; Sumida, Y.; Kanamori, K.; Yoshimura, K.; Hayashi, N.; Muranaka, S.; Takano, M.; et al. Spin-ladder iron oxide: Sr3Fe2O5. Angew. Chem. Int. Ed. Engl. 2008, 47, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.A.; Green, M.A.; Rosseinsky, M.J.; Sloan, J. Sodium Hydride as a Powerful Reducing Agent for Topotactic Oxide Deintercalation: Synthesis and Characterization of the Nickel (I) Oxide LaNiO2. J. Am. Chem. Soc. 1999, 121, 8843–8854. [Google Scholar] [CrossRef]
- Overton, A.J.; Best, J.L.; Saratovsky, I.; Hayward, M.A. Influence of topotactic reduction on the structure and magnetism of the multiferroic YMnO3. Chem. Mater. 2009, 21, 4940–4948. [Google Scholar] [CrossRef]
- Hayward, M.A.; Cussen, E.J.; Claridge, J.B.; Bieringer, M.; Rosseinsky, M.J.; Kiely, C.J.; Blundell, S.J.; Marshall, I.M.; Pratt, F.L. The Hydride Anion in an Extended Transition Metal Oxide Array: LaSrCoO3H0.7. Science 2002, 295, 1882–1884. [Google Scholar] [CrossRef] [PubMed]
- Lindemer, T.B.; Hunley, J.F.; Gates, J.E.; Sutton, A.L.F.; Brynestad, J.; Hubbard, C.R.; Gallagher, P.K. Experimental and Thermodynamic Study of Nonstoichiometry in YBa2Cu3O7−x. J. Am. Ceram. Soc. 1989, 72, 1775–1788. [Google Scholar] [CrossRef]
- Miyoshi, S.; Hong, J.O.; Yashiro, K.; Kaimai, A.; Nigara, Y.; Kawamura, K.; Kawada, T.; Kawada, J. Lattice expansion upon reduction of perovskite-type LaMnO3 with oxygen-deficit nonstoichiometry. Solid State Ion. 2003, 161, 209–217. [Google Scholar] [CrossRef]
- Mizusaki, J.; Yoshihiro, M.; Yamauchi, S.; Fueki, K. Nonstoichiometry and Defect Structure of the Perovskite-Type Oxides La1−xSrxFeO3−d. J. Solid State Chem. 1985, 58, 257–266. [Google Scholar] [CrossRef]
- Uusi-Esko, K.; Rautama, E.L.; Laitinen, M.; Sajavaara, T.; Karppinen, M. Control of Oxygen Nonstoichiometry and Magnetic Property of MnCo2O4 Thin Films Grown by Atomic Layer Deposition. Chem. Mater. 2010, 22, 6297–6300. [Google Scholar] [CrossRef]
- Feteira, A. Negative temperature coefficient resistance (NTCR) ceramic thermistors: An industrial perspective. J. Am. Ceram. Soc. 2009, 92, 967–983. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Zhecheva, E.; Ivanov, G.; Ioncheva, R. Preparation and catalytic activity of nickel-manganese oxide catalysts with an ilmenite-type structure in the reactions of complete oxidation of hydrocarbons. Appl. Catal. A 1998, 167, 277–282. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Naydenov, A.; Ivanov, G. Ozone decomposition, benzene and CO oxidation over NiMnO3-ilmenite and NiMn2O4-spinel catalysts. Appl. Catal. A 2001, 206, 13–18. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D.; Nabavi, S.R. Nanocrystalline AMn2O4 (A=Co, Ni, Cu) spinels for remediation of volatile organic compounds-synthesis, characterization and catalytic performance. Ceram. Int. 2012, 38, 1655–1661. [Google Scholar] [CrossRef]
- Nelson-Cheeseman, B.B.; Chopdekar, R.V.; Alldredge, L.M.B.; Bettinger, J.S.; Arenholz, E.; Suzuki, Y. Probing the role of the barrier layer in magnetic tunnel junction transport. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Nelson-Cheeseman, B.B.; Chopdekar, R. V.; Iwata, J.M.; Toney, M.F.; Arenholz, E.; Suzuki, Y. Modified magnetic ground state in NiMn2O4 thin films. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Lisboa-Filho, P.N.; Bahout, M.; Barahona, P.; Moure, C.; Peña, O. Oxygen stoichiometry effects in spinel-type NiMn2O4−δ samples. J. Phys. Chem. Solids 2005, 66, 1206–1212. [Google Scholar] [CrossRef]
- Tadic, M.; Savic, S.M.; Jaglicic, Z.; Vojisavljevic, K.; Radojkovic, A.; Prsic, S.; Nikolic, D. Magnetic properties of NiMn2O4−δ (nickel manganite): Multiple magnetic phase transitions and exchange bias effect. J. Alloy. Compd. 2014, 588, 465–469. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M. Microwave-Energy Distribution for Reduction and Decrystallization of Titanium Oxides. Chem. Lett. 2012, 41, 39–41. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M.; Sano, S.; Hayashi, Y.; Takizawa, H. In-situ kinetic study on non-thermal reduction reaction of CuO during microwave heating. Mater. Lett. 2013, 91, 252–254. [Google Scholar] [CrossRef]
- Fukushima, J.; Sato, M.; Nakamura, H. Plasma Model for Energy Transformation Mechanism of Non-Thermal Microwave Effect. Plasma Fusion Res. 2012, 7, 1–2. [Google Scholar] [CrossRef]
- Kashimura, K.; Nagata, K.; Sato, M. Concept of Furnace for Metal Refining by Microwave Heating—A Design of Microwave Smelting Furnace with Low CO2 Emission. Mater. Trans. 2010, 51, 1847–1853. [Google Scholar] [CrossRef]
- Kashimura, K.; Sato, M.; Hotta, M.; Agrawal, D.; Nagata, K.; Hayashi, M.; Mitani, T.; Shinohara, N. Iron production from Fe3O4 and graphite by applying 915MHz microwaves. Mater. Sci. Eng. A 2012, 556, 977–979. [Google Scholar] [CrossRef]
- Nagata, K.; Sato, M.; Hara, K.; Hotta, T.; Kitamura, Y.; Hayashi, M.; Kashimura, K.; Mitani, T.; Fukushima, J. Microwave Blast Furnace and Its Refractories. J. Tech. Assoc. Refract. Jpn. 2014, 34, 66–73. [Google Scholar]
- Roy, R.; Peelamedu, R.; Hurtt, L.; Cheng, J.; Agrawal, D. Definitive experimental evidence for Microwave Effects: Radically new effects of separated E and H fields, such as decrystallization of oxides in seconds. Mater. Res. Innov. 2002, 6, 128–140. [Google Scholar] [CrossRef]
- Takayama, S.; Fukushima, J.; Nishijo, J.; Saito, M.; Sano, S.; Sato, M. Sintering of Soft Magnetic Material under Microwave Magnetic Field. Phys. Res. Int. 2012, 2014, 1–4. [Google Scholar] [CrossRef]
- Kumar, P.; Juneja, J.K.; Prakash, C.; Singh, S.; Shukla, R.K.; Raina, K.K. High DC resistivity in microwave sintered Li0.49Zn0.02Mn0.06Fe2.43O4 ferrites. Ceram. Int. 2014, 40, 2501–2504. [Google Scholar] [CrossRef]
- Wickham, D.G. Solid-Phase Equilibria in the System NiO-Mn2O3-O2. J. Inorg. Nucl. Chem. 1964, 26, 1369–1377. [Google Scholar] [CrossRef]
- Shen, Y.; Nakayama, T.; Arai, M.; Yanagisawa, O.; Izumi, M. Magnetic phase transition and physical properties of spinel-type nickel manganese oxide. J. Phys. Chem. Solids 2002, 63, 947–950. [Google Scholar] [CrossRef]
- Savić, S.M.; Tadić, M.; Jagličić, Z.; Vojisavljević, K.; Mancic, L.; Branković, G. Structural, electrical and magnetic properties of nickel manganite obtained by a complex polymerization method. Ceram. Int. 2014, 40, 15515–15521. [Google Scholar] [CrossRef]
- Yafet, Y.; Kittel, C. Antiferromagnetic arrangements in ferrites. Phys. Rev. 1952, 87, 290–294. [Google Scholar] [CrossRef]
- Arroyo, R.; Cordoba, G.; Padilla, J.; Lara, V.H. Influence of manganese ions on the anatase-rutile phase transition of TiO2 prepared by the sol-gel process. Mater. Lett. 2002, 54, 397–402. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Orgel, L.E. Stereochemistry of ionic solids. Adv. Inorg. Chem. Radiochem. 1960, 2, 1–60. [Google Scholar]







| Magnetization (emu/g) (at 50000 Oe) | Coercivity (Oe) | |
|---|---|---|
| Pre-NiMnO | 36.3 ± 1.8 | 140 ± 7 |
| H-field irradiated sample | 16.5 ± 0.8 | 530 ± 27 |
| conv.-heated sample | 35.5 ± 1.8 | 95 ± 5 |
| Annealed sample | 36.0 ± 1.8 | 130 ± 7 |
| Magnetization (emu/g) (at 50000 Oe) | Coercivity (Oe) | |
|---|---|---|
| Pre-NiMnO | 35.3 | 140 |
| H-field irradiated sample(400 C) | 27.4 ± 1.4 | 280 ± 14 |
| H-field irradiated sample(500 C) | 23.5 ± 1.2 | 480 ± 24 |
| H-field irradiated sample(600 C) | 19.7 ± 1.0 | 630 ± 32 |
| H-field irradiated sample(700 C) | 21.6 ± 1.1 | 750 ± 38 |
| H-field irradiated sample(800 C) | 18.2 ± 0.9 | 750 ± 38 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, H.; Fukushima, J.; Takizawa, H. Control of Magnetic Properties of NiMn2O4 by a Microwave Magnetic Field under Air. Materials 2016, 9, 169. https://doi.org/10.3390/ma9030169
Goto H, Fukushima J, Takizawa H. Control of Magnetic Properties of NiMn2O4 by a Microwave Magnetic Field under Air. Materials. 2016; 9(3):169. https://doi.org/10.3390/ma9030169
Chicago/Turabian StyleGoto, Hiroshi, Jun Fukushima, and Hirotsugu Takizawa. 2016. "Control of Magnetic Properties of NiMn2O4 by a Microwave Magnetic Field under Air" Materials 9, no. 3: 169. https://doi.org/10.3390/ma9030169
APA StyleGoto, H., Fukushima, J., & Takizawa, H. (2016). Control of Magnetic Properties of NiMn2O4 by a Microwave Magnetic Field under Air. Materials, 9(3), 169. https://doi.org/10.3390/ma9030169