MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells
Abstract
:1. Introduction
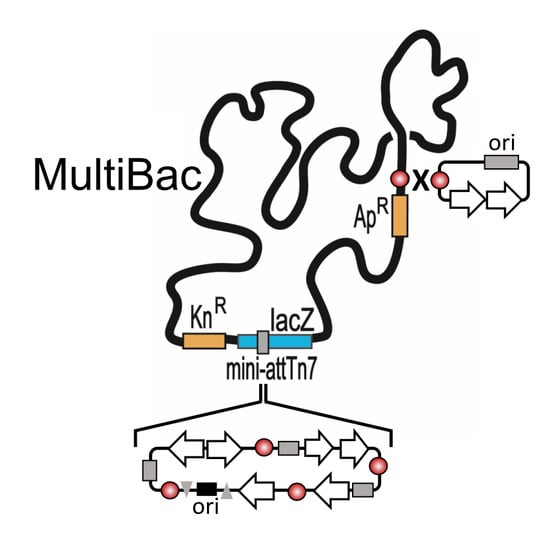
2. The MultiBac BICS: Enabling Multiprotein Complex Structure Analysis
3. Mechanisms of Transcription Factor Complex Assembly
4. G-Protein Coupled Receptor (GPCR) Structure and Mechanism
5. VLP-FactoryTM: Customized MultiBac Baculovirus for Virus-Like Particle Production
6. MultiBacMam-BiFC: Cell-Based Screening by Bimolecular Fluorescence Complementation
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of human β interferon in insect cells infected with a baculovirus expression vector. Mol. Cell Biol. 1983, 3, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.D. Milestones leading to the genetic engineering of baculoviruses as expression vector systems and viral pesticides. Adv. Virus Res. 2006, 68, 3–73. [Google Scholar] [PubMed]
- Boyce, F.M.; Bucher, N.L. Baculovirus-mediated gene transfer into mammalian cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2348–2352. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, L.F.; Klowden, M.J.; Miller, L.K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J. Virol. 1985, 56, 153–160. [Google Scholar] [PubMed]
- Hofmann, C.; Sandig, V.; Jennings, G.; Rudolph, M.; Schlag, P.; Strauss, M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc. Natl. Acad. Sci. USA 1995, 92, 10099–10103. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Aksular, M.; Graves, L.P.; Irons, S.L.; Possee, R.D.; King, L.A. Overview of the baculovirus expression system. Curr. Protoc. Protein Sci. 2018, 91, 5.4.1–5.4.6. [Google Scholar] [PubMed]
- Irons, S.L.; Chambers, A.C.; Lissina, O.; King, L.A.; Possee, R.D. Protein production using the baculovirus expression system. Curr. Protoc. Protein Sci. 2018, 91, 5.5.1–5.5.22. [Google Scholar] [PubMed]
- Mansouri, M.; Berger, P. Baculovirus for gene delivery to mammalian cells: Past, present and future. Plasmid 2018, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Premanand, B.; Zhong Wee, P.; Prabakaran, M. Baculovirus surface display of immunogenic proteins for vaccine development. Viruses 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J. Gen. Virol. 2015, 96, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Fitzgerald, D.J.; Richmond, T.J. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004, 22, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Bieniossek, C.; Richmond, T.J.; Berger, I. MultiBac: Multigene baculovirus-based eukaryotic protein complex production. Curr. Protoc. Protein. Sci. 2008. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Berger, P.; Schaffitzel, C.; Yamada, K.; Richmond, T.J.; Berger, I. Protein complex expression by using multigene baculoviral vectors. Nat. Methods 2006, 3, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Garzoni, F.; Chaillet, M.; Haffke, M.; Gupta, K.; Aubert, A. The multibac protein complex production platform at the EMBL. J. Vis. Exp. 2013, 77, e50159. [Google Scholar] [CrossRef] [PubMed]
- Bieniossek, C.; Imasaki, T.; Takagi, Y.; Berger, I. MultiBac: Expanding the research toolbox for multiprotein complexes. Trends Biochem. Sci. 2012, 37, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Viola, C.; Bieniossek, C.; Trowitzsch, S.; Vijay-Achandran, L.S.; Chaillet, M.; Garzoni, F.; Berger, I. Getting a grip on complexes. Curr. Genom. 2009, 10, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Palmberger, D.; Klausberger, M.; Berger, I.; Grabherr, R. MultiBac turns sweet. Bioengineered 2013, 4, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosse, M.; Crocker, H.; Gorda, B.; Lemaire, P.; Rauch, J.; Berger, I. MultiBac: From protein complex structures to synthetic viral nanosystems. BMC Biol. 2017, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.; Gupta, K.; Thimiri Govinda Raj, D.B.; Aubert, A.; Drncova, P.; Garzoni, F.; Fitzgerald, D.; Berger, I. The multibac baculovirus/insect cell expression vector system for producing complex protein biologics. Adv. Exp. Med. Biol. 2016, 896, 199–215. [Google Scholar] [PubMed]
- Trowitzsch, S.; Bieniossek, C.; Nie, Y.; Garzoni, F.; Berger, I. New baculovirus expression tools for recombinant protein complex production. J. Struct. Biol. 2010, 172, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Trowitzsch, S.; Palmberger, D.; Fitzgerald, D.; Takagi, Y.; Berger, I. MultiBac complexomics. Expert Rev. Proteomics 2012, 9, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Vijayachandran, L.S.; Thimiri Govinda Raj, D.B.; Edelweiss, E.; Gupta, K.; Maier, J.; Gordeliy, V.; Fitzgerald, D.J.; Berger, I. Gene gymnastics: Synthetic biology for baculovirus expression vector system engineering. Bioengineered 2013, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Vijayachandran, L.S.; Viola, C.; Garzoni, F.; Trowitzsch, S.; Bieniossek, C.; Chaillet, M.; Schaffitzel, C.; Busso, D.; Romier, C.; Poterszman, A.; et al. Robots, pipelines, polyproteins: Enabling multiprotein expression in prokaryotic and eukaryotic cells. J. Struct. Biol. 2011, 175, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.V.; Sali, A.; Baumeister, W. The molecular sociology of the cell. Nature 2007, 450, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Luckow, V.A.; Lee, S.C.; Barry, G.F.; Olins, P.O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [PubMed]
- Luckow, V.A. Baculovirus systems for the expression of human gene products. Curr. Opin. Biotechnol. 1993, 4, 564–572. [Google Scholar] [CrossRef]
- Trowitzsch, S.; Klumpp, M.; Thoma, R.; Carralot, J.P.; Berger, I. Light it up: Highly efficient multigene delivery in mammalian cells. Bioessays 2011, 33, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Bieniossek, C.; Nie, Y.; Frey, D.; Olieric, N.; Schaffitzel, C.; Collinson, I.; Romier, C.; Berger, P.; Richmond, T.J.; Steinmetz, M.O.; et al. Automated unrestricted multigene recombineering for multiprotein complex production. Nat. Methods 2009, 6, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Schaffitzel, C.; Berger, P.; Wellinger, R.; Bieniossek, C.; Richmond, T.J.; Berger, I. Multiprotein expression strategy for structural biology of eukaryotic complexes. Structure 2007, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Haffke, M.; Viola, C.; Nie, Y.; Berger, I. Tandem recombineering by SLIC cloning and Cre-LoxP fusion to generate multigene expression constructs for protein complex research. Methods Mol. Biol. 2013, 1073, 131–140. [Google Scholar] [PubMed]
- Nie, Y.; Chaillet, M.; Becke, C.; Haffke, M.; Pelosse, M.; Fitzgerald, D.; Collinson, I.; Schaffitzel, C.; Berger, I. Acembl tool-kits for high-throughput multigene delivery and expression in prokaryotic and eukaryotic hosts. Adv. Exp. Med. Biol. 2016, 896, 27–42. [Google Scholar] [PubMed]
- Thimiri Govinda Raj, D.B.; Vijayachandran, L.S.; Berger, I. Omnibac: Universal multigene transfer plasmids for baculovirus expression vector systems. Methods Mol. Biol. 2014, 1091, 123–130. [Google Scholar] [PubMed]
- Weissmann, F.; Petzold, G.; VanderLinden, R.; Huis In ’t Veld, P.J.; Brown, N.G.; Lampert, F.; Westermann, S.; Stark, H.; Schulman, B.A.; Peters, J.M. Bigbac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proc. Natl. Acad. Sci. USA 2016, 113, E2564–E2569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, J.; Barford, D. Recombinant expression and reconstitution of multiprotein complexes by the user cloning method in the insect cell-baculovirus expression system. Methods 2016, 95, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Stolt-Bergner, P.; Benda, C.; Bergbrede, T.; Besir, H.; Celie, P.H.N.; Chang, C.; Drechsel, D.; Fischer, A.; Geerlof, A.; Giabbai, B.; et al. Baculovirus-driven protein expression in insect cells: A benchmarking study. J. Struct. Biol. 2018, 203, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Aramayo, R.J.; Willhoft, O.; Ayala, R.; Bythell-Douglas, R.; Wigley, D.B.; Zhang, X. Cryo-EM structures of the human INO80 chromatin-remodeling complex. Nat. Struct. Mol. Biol. 2018, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ayala, R.; Willhoft, O.; Aramayo, R.J.; Wilkinson, M.; McCormack, E.A.; Ocloo, L.; Wigley, D.B.; Zhang, X. Structure and regulation of the human INO80-nucleosome complex. Nature 2018, 556, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Eustermann, S.; Schall, K.; Kostrewa, D.; Lakomek, K.; Strauss, M.; Moldt, M.; Hopfner, K.P. Structural basis for ATP-dependent chromatin remodelling by the INO80 complex. Nature 2018, 556, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Knoll, K.R.; Eustermann, S.; Niebauer, V.; Oberbeckmann, E.; Stoehr, G.; Schall, K.; Tosi, A.; Schwarz, M.; Buchfellner, A.; Korber, P.; et al. The nuclear actin-containing Arp8 module is a linker DNA sensor driving INO80 chromatin remodeling. Nat. Struct. Mol. Biol. 2018, 25, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.M.; Willhoft, O.; Lin, C.L.; Bythell-Douglas, R.; Wigley, D.B. Production and assay of recombinant multisubunit chromatin remodeling complexes. Methods Enzymol. 2017, 592, 27–47. [Google Scholar] [PubMed]
- Willhoft, O.; Ghoneim, M.; Lin, C.L.; Chua, E.Y.D.; Wilkinson, M.; Chaban, Y.; Ayala, R.; McCormack, E.A.; Ocloo, L.; Rueda, D.S.; et al. Structure and dynamics of the yeast SWR1-nucleosome complex. Science 2018, 362, eaat7716. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.M.; Farnung, L.; Boehning, M.; Wigge, C.; Linden, A.; Urlaub, H.; Cramer, P. Structure of activated transcription complex Pol II–DSIF–PAF–SPT6. Nature 2018, 560, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Pentakota, S.; Zhou, K.; Smith, C.; Maffini, S.; Petrovic, A.; Morgan, G.P.; Weir, J.R.; Vetter, I.R.; Musacchio, A.; Luger, K. Decoding the centromeric nucleosome through CENP-N. Elife 2017, 6, e33442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Hamilton, K.; Manley, J.L.; Shi, Y.; Walz, T.; Tong, L. Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proc. Natl. Acad. Sci. USA 2018, 115, E1419–E1428. [Google Scholar] [CrossRef] [PubMed]
- Tarbouriech, N.; Ducournau, C.; Hutin, S.; Mas, P.J.; Man, P.; Forest, E.; Hart, D.J.; Peyrefitte, C.N.; Burmeister, W.P.; Iseni, F. The vaccinia virus DNA polymerase structure provides insights into the mode of processivity factor binding. Nat. Commun. 2017, 8, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basters, A.; Geurink, P.P.; Rocker, A.; Witting, K.F.; Tadayon, R.; Hess, S.; Semrau, M.S.; Storici, P.; Ovaa, H.; Knobeloch, K.P.; et al. Structural basis of the specificity of USP18 toward ISG15. Nat. Struct. Mol. Biol. 2017, 24, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, S.; Tong, L. Molecular mechanism for the regulation of yeast separase by securin. Nature 2017, 542, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, W.C.; Murayama, Y.; Munoz, S.; Jones, A.W.; Wade, B.O.; Purkiss, A.G.; Hu, X.W.; Borg, A.; Snijders, A.P.; Uhlmann, F.; et al. Structure of the cohesin loader Scc2. Nat. Commun. 2017, 8, 13952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Sari-Ak, D.; Haffke, M.; Trowitzsch, S.; Berger, I. Zooming in on transcription preinitiation. J. Mol. Biol. 2016, 428, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Antonova, S.V.; Haffke, M.; Corradini, E.; Mikuciunas, M.; Low, T.Y.; Signor, L.; van Es, R.M.; Gupta, K.; Scheer, E.; Vos, H.R.; et al. Chaperonin CCT checkpoint function in basal transcription factor TFIID assembly. Nat. Struct. Mol. Biol. 2018, 25, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Bieniossek, C.; Papai, G.; Schaffitzel, C.; Garzoni, F.; Chaillet, M.; Scheer, E.; Papadopoulos, P.; Tora, L.; Schultz, P.; Berger, I. The architecture of human general transcription factor TFIID core complex. Nature 2013, 493, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Watson, A.A.; Baptista, T.; Scheer, E.; Chambers, A.L.; Koehler, C.; Zou, J.; Obong-Ebong, I.; Kandiah, E.; Temblador, A.; et al. Architecture of TAF11/TAF13/TBP complex suggests novel regulation properties of general transcription factor TFIID. Elife 2017, 6, e30395. [Google Scholar] [CrossRef] [PubMed]
- Trowitzsch, S.; Viola, C.; Scheer, E.; Conic, S.; Chavant, V.; Fournier, M.; Papai, G.; Ebong, I.O.; Schaffitzel, C.; Zou, J.; et al. Cytoplasmic taf2-taf8-taf10 complex provides evidence for nuclear holo-tfiid assembly from preformed submodules. Nat. Commun. 2015, 6, 6011. [Google Scholar] [CrossRef] [PubMed]
- Koehler, C.; Sauter, P.F.; Wawryszyn, M.; Girona, G.E.; Gupta, K.; Landry, J.J.; Fritz, M.H.; Radic, K.; Hoffmann, J.E.; Chen, Z.A.; et al. Genetic code expansion for multiprotein complex engineering. Nat. Methods 2016, 13, 997–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Bellon-Echeverria, I.; Trowitzsch, S.; Bieniossek, C.; Berger, I. Multiprotein complex production in insect cells by using polyproteins. Methods Mol. Biol. 2014, 1091, 131–141. [Google Scholar] [PubMed]
- Crepin, T.; Swale, C.; Monod, A.; Garzoni, F.; Chaillet, M.; Berger, I. Polyproteins in structural biology. Curr. Opin. Struct. Biol. 2015, 32, 139–146. [Google Scholar] [CrossRef] [PubMed]
- El-Saafin, F.; Curry, C.; Ye, T.; Garnier, J.M.; Kolb-Cheynel, I.; Stierle, M.; Downer, N.L.; Dixon, M.P.; Negroni, L.; Berger, I.; et al. Homozygous taf8 mutation in a patient with intellectual disability results in undetectable TAF8 protein, but preserved RNA polymerase II transcription. Hum. Mol. Genet. 2018, 27, 2171–2186. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.C.M. Building transcription complexes. Nat. Struct. Mol. Biol. 2019, 26, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Braunagel, S.C.; Summers, M.D. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr. Drug Targets 2007, 8, 1084–1095. [Google Scholar] [CrossRef]
- Carpenter, B.; Tate, C.G. Active state structures of g protein-coupled receptors highlight the similarities and differences in the g protein and arrestin coupling interfaces. Curr. Opin. Struct. Biol. 2017, 45, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556. [Google Scholar] [CrossRef] [PubMed]
- Munk, C.; Mutt, E.; Isberg, V.; Nikolajsen, L.F.; Bibbe, J.M.; Flock, T.; Hanson, M.A.; Stevens, R.C.; Deupi, X.; Gloriam, D.E. An online resource for GPCR structure determination and analysis. Nat. Methods 2019, 16, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Shimada, I.; Ueda, T.; Kofuku, Y.; Eddy, M.T.; Wuthrich, K. GPCR drug discovery: Integrating solution NMR data with crystal and cryo-EM structures. Nat. Rev. Drug Discov. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.M.; Vuckovic, Z.; Draper-Joyce, C.J.; Liang, Y.L.; Glukhova, A.; Christopoulos, A.; Sexton, P.M. Recent advances in the determination of g protein-coupled receptor structures. Curr. Opin. Struct. Biol. 2018, 51, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Stevens, R.C.; Roth, B.L. How ligands illuminate GPCR molecular pharmacology. Cell 2017, 170, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Ehrenmann, J.; Schoppe, J.; Klenk, C.; Rappas, M.; Kummer, L.; Dore, A.S.; Pluckthun, A. High-resolution crystal structure of parathyroid hormone 1 receptor in complex with a peptide agonist. Nat. Struct. Mol. Biol. 2018, 25, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Chau, B.; Rodgers, L.; Lu, F.; Wilbur, K.L.; Otto, K.A.; Chen, Y.; Song, M.; Riley, J.P.; Yang, H.C.; et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat. Commun. 2018, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Schoppe, J.; Ehrenmann, J.; Klenk, C.; Rucktooa, P.; Schutz, M.; Dore, A.S.; Pluckthun, A. Crystal structures of the human neurokinin 1 receptor in complex with clinically used antagonists. Nat. Commun. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Hillenbrand, M.; Schori, C.; Schoppe, J.; Pluckthun, A. Comprehensive analysis of heterotrimeric G-protein complex diversity and their interactions with gpcrs in solution. Proc. Natl. Acad. Sci. USA 2015, 112, E1181–E1190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pluckthun, A. In vivo assembly and large-scale purification of a GPCR-Gα fusion with Gβγ, and characterization of the active complex. PLoS ONE 2019, 14, e0210131. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S. Influenza, evolution, and the next pandemic. Evol. Med. Public Health 2018, 2018, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Mirzaei, H.; Salemi, M.; Momeni, F.; Mousavi, M.J.; Sadeghalvad, M.; Arjeini, Y.; Solaymani-Mohammadi, F.; Sadri Nahand, J.; Namdari, H.; et al. Influenza vaccine: Where are we and where do we go? Rev. Med. Virol. 2019, 29, e2014. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Lee, Y.T.; Kim, K.H.; Kim, M.C.; Kang, S.M. Progress in developing virus-like particle influenza vaccines. Expert Rev. Vaccines 2016, 15, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Roldao, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Sari-Ak, D.; Bahrami, S.; Laska, M.J.; Drnkova, P.; Fitzgerald, D.J.; Schaffitzel, C.; Garzoni, F.; Berger, I. High-throughput production of influenza virus-like particle (VLP) array by using VLP-factory, a MultiBac baculoviral genome customized for enveloped VLP expression. Methods Mol. Biol. 2019, in press. [Google Scholar]
- Bahrami, S.; Laska, M.J.; Pedersen, F.S.; Duch, M. Immune suppressive activity of the influenza fusion peptide. Virus Res. 2016, 211, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Bellon-Echeverria, I.; Rizk, A.; Ehsaei, Z.; Cianciolo Cosentino, C.; Silva, C.S.; Xie, Y.; Boyce, F.M.; Davis, M.W.; Neuhauss, S.C.; et al. Highly efficient baculovirus-mediated multigene delivery in primary cells. Nat. Commun. 2016, 7, 11529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellon-Echeverria, I.; Carralot, J.P.; Del Rosario, A.A.; Kueng, S.; Mauser, H.; Schmid, G.; Thoma, R.; Berger, I. MultiBacMam Bimolecular Fluorescence Complementation (BiFC) tool-kit identifies new small-molecule inhibitors of the CDK5-p25 protein-protein interaction (PPI). Sci. Rep. 2018, 8, 5083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerppola, T.K. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 2006, 1, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonn, P.; Landegren, U. Close encounters-probing proximal proteins in live or fixed cells. Trends Biochem. Sci. 2017, 42, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Kim, Y.; Huh, W.K.; Park, H.O. Bimolecular fluorescence complementation (BiFC) analysis: Advances and recent applications for genome-wide interaction studies. J. Mol. Biol. 2015, 427, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Camins, A.; Verdaguer, E.; Folch, J.; Canudas, A.M.; Pallas, M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, G.; Greig, N.H.; Anwar, F.; Al-Abbasi, F.A.; Zamzami, M.A.; Al-Talhi, H.A.; Kamal, M.A. Neuroprotective mechanisms mediated by CDK5 inhibition. Curr. Pharm. Des. 2016, 22, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Skuntz, S.; Pant, H.C. Deregulated CDK5 activity is involved in inducing Alzheimer’s disease. Arch. Med. Res. 2012, 43, 655–662. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, K.; Tölzer, C.; Sari-Ak, D.; Fitzgerald, D.J.; Schaffitzel, C.; Berger, I. MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells. Viruses 2019, 11, 198. https://doi.org/10.3390/v11030198
Gupta K, Tölzer C, Sari-Ak D, Fitzgerald DJ, Schaffitzel C, Berger I. MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells. Viruses. 2019; 11(3):198. https://doi.org/10.3390/v11030198
Chicago/Turabian StyleGupta, Kapil, Christine Tölzer, Duygu Sari-Ak, Daniel J. Fitzgerald, Christiane Schaffitzel, and Imre Berger. 2019. "MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells" Viruses 11, no. 3: 198. https://doi.org/10.3390/v11030198
APA StyleGupta, K., Tölzer, C., Sari-Ak, D., Fitzgerald, D. J., Schaffitzel, C., & Berger, I. (2019). MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells. Viruses, 11(3), 198. https://doi.org/10.3390/v11030198