siRNA for Influenza Therapy
Abstract
:1. Introduction
4. Inhibition of Influenza by siRNA Targeting Viral mRNA
5. Inhibition of Influenza by siRNA Targeting Cellular mRNA
6. Testing Anti-influenza siRNA
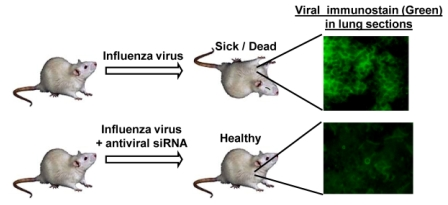
7. Anti-influenza siRNA Testing in an Animal Model
8. Advantages of siRNA as Anti-influenza Drug
9. Conclusion: The Future of Anti-influenza siRNA
| Target viral mRNA (nt #) | siRNA sequence: top strand 5' to 3' |
|---|---|
| PB2-2210 | GGAGACGUGGUGUUGGUAAdTdT dTdTCCUCUGCACCACAACCAUU |
| PB2-2240 | CGGGACUCUAGCAUACUUAdTdT dTdTGCCCUGAGAUCGUAUGAAU |
| PB1-6 | GCAGGCAAACCAUUUGAAUdTdT dTdTCGUCCGUUUGGUAAACUUA |
| PB1-129 | CAGGAUACACCAUGGAUACdTdT dTdTGUCCUAUGUGGUACCUAUG |
| PB1-2257 | GAUCUGUUCCACCAUUGAAdTdT dTdTCUAGACAAGGUGGUAACUU |
| PA-44 | UGCUUCAAUCCGAUGAUUGdTdT dTdTACGAAGUUAGGCUACUAAC |
| PA-739 | CGGCUACAUUGAGGGCAAGdTdT dTdTGCCGAUGUAACUCCCGUUC |
| PA-2087 | GCAAUUGAGGAGUGCCUGAdTdT dTdTCGUUAACUCCUCACGGACU |
| PA-2110 | UGAUCCCUGGGUUUUGCUUdTdT dTdTACUAGGGACCCAAAACGAA |
| PA-2131 | UGCUUCUUGGUUCAACUCCdTdT dTdTACGAAGAACCAAGUUGAGG |
| NP-231 | UAGAGAGAAUGGUGCUCUCdTdT dTdTAUCUCUCUUACCACGAGAG |
| NP-390 | UAAGGCGAAUCUGGCGCCAdTdT dTdTAUUCCGCUUAGACCGCGGU |
| NP-1496 | GGAUCUUAUUUCUUCGGAGdTdT dTdTCCUAGAAUAAAGAAGCCUC |
| M-37 | CCGAGGUCGAAACGUACGUdTdT dTdTGGCUCCAGCUUUGCAUGCA |
| M-480 | CAGAUUGCUGACUCCCAGCdTdT dTdTGUCUAACGACUGAGGGUCG |
| M-598 | UGGCUGGAUCGAGUGAGCAdTdT dTdTACCGACCUAGCUCACUCGU |
| M-934 | GAAUAUCGAAAGGAACAGCdTdT dTdTCUUAUAGCUUUCCUUGUCG |
| NS-128 | CGGCUUCGCCGAGAUCAGAdAdT dTdAGCCGAAGCGGCUCUAGUCU |
| NS-562 | GUCCUCCGAUGAGGACUCCdTdT dTdTCAGGAGGCUACUCCUGAGG |
| NS-589 | UGAUAACACAGUUCGAGUCdTdT dTdTACUAUUGUGUCAAGCUCAG |
Acknowledgments
References
- Saladino, R.; Barontini, M.; Crucianelli, M.; Nencioni, L.; Sgarbanti, R.; Palamara, A.T. Current Advances in Anti-Influenza Therapy . Curr. Med. Chem. 2010. [Google Scholar]
- Schmolke, M.; García-Sastre, A. Evasion of innate and adaptive immune responses by influenza A virus. Cell. Microbiol. 2010. [Google Scholar]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses . Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Tumpey, T.M.; Belser, J.A. Resurrected pandemic influenza viruses. Annu. Rev. Microbiol. 2009, 63, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Musiyenko, A.; Barik, S. Viral infection of the lungs through the eye . J. Virol. 2007, 81, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.Z. Gene silencing by double-stranded RNA. Cell Death Differ. 2007, 14, 1998–2012. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Barik, S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; McManus, M.T.; Nguyen, T.; Shen, C.H.; Sharp, P.A.; Eisen, H.N.; Chen, J. RNA interference of influenza virus production by directly targeting mRNA for degradation and indirectly inhibiting all viral RNA transcription. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Control of nonsegmented negative-strand RNA virus replication by siRNA. Virus Res. 2004, 102, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Filip, L.; Bai, A.; Nguyen, T.; Eisen, H.N.; Chen, J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 8676–8681. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, S.M.; Lo, C.Y.; Tumpey, T.M.; Epstein, S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 8682–8686. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.J. The use of RNAi-based screens to identify host proteins involved in viral replication. Future Microbiol. 2010, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.K.; Kim, M.; Reche, P.A.; Morehead, T.J.; Damon, I.K.; Welsh, R.M.; Reinherz, E.L. Antiviral chemotherapy facilitates control of poxvirus infections through inhibition of cellular signal transduction. J. Clin. Invest. 2005, 115, 379–387. [Google Scholar] [PubMed]
- Briz, V.; Poveda, E.; Soriano, V. HIV entry inhibitors: mechanisms of action and resistance pathways. J. Antimicrob. Chemother. 2006, 57, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Sakurai, A.; Watanabe, T.; Sorensen, E.; Nidom, C.A.; Newton, M.A.; Ahlquist, P.; Kawaoka, Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 2008, 454, 890–893. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Stertz, S.; Zhou, Y.; Inoue, A.; Hoffmann, H.H.; Bhattacharyya, S.; Alamares, J.G.; Tscherne, D.M.; Ortigoza, M.B.; Liang, Y.; Gao, Q.; Andrews, S.E.; Bandyopadhyay, S.; De Jesus, P.; Tu, B.P.; Pache, L.; Shih, C.; Orth, A.; Bonamy, G.; Miraglia, L.; Ideker, T.; García-Sastre, A.; Young, J.A.; Palese, P.; Shaw, M.L.; Chanda, S.K. Human host factors required for influenza virus replication. Nature 2010, 463, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Karlas, A.; Machuy, N.; Shin, Y.; Pleissner, K.P.; Artarini, A.; Heuer, D.; Becker, D.; Khalil, H.; Ogilvie, L.A.; Hess, S.; Mäurer, A.P.; Müller, E.; Wolff, T.; Rudel, T.; Meyer, T.F. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 2010, 463, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Satterly, N.; Tsai, P.L.; van Deursen, J.; Nussenzveig, D.R.; Wang, Y.; Faria, P.A.; Levay, A.; Levy, D.E.; Fontoura, B.M. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl Acad. Sci. U. S. A. 2007, 104, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Elton, D.; Simpson-Holley, M.; Archer, K.; Medcalf, L.; Hallam, R.; McCauley, J.; Digard, P. Interaction of the influenza virus nucleoprotein with the cellular CRM1-mediated nuclear export pathway. J. Virol. 2001, 75, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Harborth, J.; Elbashir, S.M.; Bechert, K.; Tuschl, T.; Weber, K. Identification of essential genes in cultured mammalian cells using small interfering RNAs . J. Cell. Sci. 2001, 114 (Pt 24), 4557–4565. [Google Scholar] [PubMed]
- Aszódi, A.; Pfeifer, A.; Ahmad, M.; Glauner, M.; Zhou, X.H.; Ny, L.; Andersson, K.E.; Kehrel, B.; Offermanns, S.; Fässler, R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Nix, D.A.; Benson, B.; Boot-Hanford, R.; Gustafsson, E.; Jamora, C.; Menzies, A.S.; Goh, K.L.; Jensen, C.C.; Gertler, F.B.; Fuchs, E.; Fassler, R.; Beckerle, M.C. Targeted disruption of the murine zyxin gene. Mol. Cell. Biol. 2003, 23, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Musiyenko, A.; Bitko, V.; Barik, S. RNAi-dependent and -independent antiviral phenotypes of chromosomally integrated shRNA clones: role of VASP in respiratory syncytial virus growth. J. Mol. Med. 2007, 85, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Harpen, M.; Barik, T.; Musiyenko, A.; Barik, S. Mutational analysis reveals a noncontractile but interactive role of actin and profilin in viral RNA-dependent RNA synthesis. J. Virol. 2009, 83, 10869–10876. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Oldenburg, A.; Garmon, N.E.; Barik, S. Profilin is required for viral morphogenesis, syncytium formation, and cell-specific stress fiber induction by respiratory syncytial virus. BMC Microbiol. 2003, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, E.; Mahoney, N.M.; Almo, S.C.; Barik, S. Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J. Virol. 2000, 74, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Templin, M.V.; Severson, G.; Baturevych, O. A potential therapeutic for pandemic influenza using RNA interference. Methods Mol. Biol. 2010, 623, 397–422. [Google Scholar] [PubMed]
- Ge, Q.; Eisen, H.N.; Chen, J. Use of siRNAs to prevent and treat influenza virus infection. Virus Res. 2004, 102, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bitko, V.; Musiyenko, A.; Shulyayeva, O.; Barik, S. Inhibition of respiratory viruses by nasally administered siRNA . Nat. Med. 2005, 11, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Treating respiratory viral diseases with chemically modified, second generation intranasal siRNAs. Methods Mol. Biol. 2009, 487, 331–341. [Google Scholar] [PubMed]
- Hornung, V.; Guenthner-Biller, M.; Bourquin, C.; Ablasser, A.; Schlee, M.; Uematsu, S.; Noronha, A.; Manoharan, M.; Akira, S.; de Fougerolles, A.; Endres, S.; Hartmann, G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005, 11, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Judge, A.; MacLachlan, I. Overcoming the innate immune response to small interfering RNA. Hum. Gene Ther. 2008, 19, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Tang, Q.; Cheng, D.; Qin, C.; Xie, F.Y.; Wei, Q.; Xu, J.; Liu, Y.; Zheng, B.J.; Woodle, M.C.; Zhong, N.; Lu, P.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005, 11, 944–951. [Google Scholar] [PubMed]
- Gaglione, M.; Messere, A. Recent progress in chemically modified siRNAs. Mini Rev. Med. Chem. 2010, 10, 578–595. [Google Scholar]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery . Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Gantier, M.P.; Tong, S.; Behlke, M.A.; Irving, A.T.; Lappas, M.; Nilsson, U.W.; Latz, E.; McMillan, N.A. Rational design of immunostimulatory siRNAs . Mol. Ther. 2010, 18, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Cochrane, M.; Leggatt, G.R.; Payne, E.; Choyce, A.; Zhou, F.; Tindle, R.; McMillan, N.A. Both treated and untreated tumors are eliminated by short hairpin RNA-based induction of target-specific immune responses. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 8314–8319. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Barik, S. siRNA for Influenza Therapy. Viruses 2010, 2, 1448-1457. https://doi.org/10.3390/v2071448
Barik S. siRNA for Influenza Therapy. Viruses. 2010; 2(7):1448-1457. https://doi.org/10.3390/v2071448
Chicago/Turabian StyleBarik, Sailen. 2010. "siRNA for Influenza Therapy" Viruses 2, no. 7: 1448-1457. https://doi.org/10.3390/v2071448
APA StyleBarik, S. (2010). siRNA for Influenza Therapy. Viruses, 2(7), 1448-1457. https://doi.org/10.3390/v2071448