Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.1.1. Synthesis and Functionalization of Mesoporous Materials
2.1.2. Sulindac Adsorption Studies
2.2. Characterization Methods
2.2.1. Powder X-ray Diffraction (XRD)
2.2.2. Differential Scanning Calorimetry (DSC)
2.2.3. Transmission and Scanning Electron Microscopy (TEM and SEM)
2.2.4. Fourier Transformed Infrared Spectroscopy (FTIR)
2.2.5. Proton Nuclear Magnetic Resonance (1H-NMR)
2.2.6. Spectrophotometry
2.3. Drug Release Studies
2.4. Cytotoxicity Studies
2.4.1. Cell Culture
2.4.2. Cell Viability
3. Results and Discussion
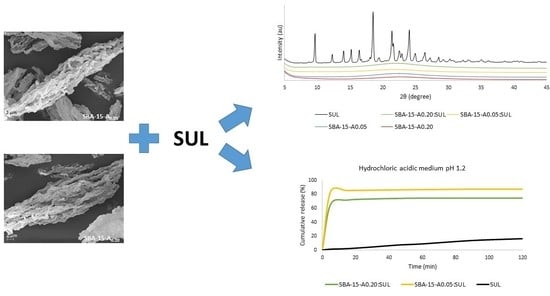
3.1. Powder X-ray Diffraction (XRD)
3.2. Differential Scanning Calorimetry (DSC)
3.3. Transmission and Scanning Electron Microscopy (TEM and SEM)
3.4. Fourier Transformed Infrared Spectroscopy (FTIR)
3.5. Proton Nuclear Magnetic Resonance 1H-NMR
3.6. Drug Release Studies
3.7. Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2021, 26, 627. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous Silica Materials: From Physico-Chemical Properties to Enhanced Dissolution of Poorly Water-Soluble Drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Formation of PVP/Nimesulide MicrospheRes. by Supercritical Antisolvent Coprecipitation. J. Supercrit. Fluids 2016, 118, 19–26. [Google Scholar] [CrossRef]
- Montes, A.; Bendel, A.; Kürti, R.; Gordillo, M.D.; Pereyra, C.; Martinez de la Ossa, E. Processing Naproxen with Supercritical CO2. J. Supercrit. Fluids 2013, 75, 21–29. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical CO2 Adsorption of Non-Steroidal Anti-Inflammatory Drugs into Biopolymer Aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Synthesis of Siliceous Materials with Micro- and Mesoporosity. Microporous Mesoporous Mater. 2007, 1–3, 26–38. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. Engl. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of Micro- and Mesoporosity in SBA-15 Materials from Adsorption Data by the NLDFT Method. J. Phys. Chem. B 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N. Modified Mesoporous Silica Nanoparticles for Enhancing Oral Bioavailability and Antihypertensive Activity of Poorly Water Soluble Valsartan. Eur. J. Pharm. Sci. 2017, 99, 152–160. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; McQuay, H.J. Single Dose Oral Sulindac for Acute Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2009, 2009, CD007540. [Google Scholar] [CrossRef]
- Mohammed, A.; Yarla, N.S.; Madka, V.; Rao, C.V. Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives. Int. J. Mol. Sci. 2018, 19, 2332. [Google Scholar] [CrossRef] [Green Version]
- Rocca, J.; Manin, S.; Hulin, A.; Aissat, A.; Verbecq-Morlot, W.; Prulière-Escabasse, V.; Wohlhuter-Haddad, A.; Epaud, R.; Fanen, P.; Tarze, A. New Use for an Old Drug: COX-Independent Anti-Inflammatory Effects of Sulindac in Models of Cystic Fibrosis. Br. J. Pharmacol. 2016, 173, 1728–1741. [Google Scholar] [CrossRef] [Green Version]
- Tros de Ilarduya, M.C.; Martín, C.; Goñi, M.M.; Martínez-Ohárriz, M.C. Solubilization and Interaction of Sulindac with Beta-Cyclodextrin in the Solid State and in Aqueous Solution. Drug Dev. Ind. Pharm. 1998, 24, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Tros de Ilarduya, M.C.; Martín, C.; Goñi, M.M.; Martínez-Ohárriz, M.C. Solubilization and Interaction of Sulindac with Polyvinylpyrrolidone K30 in the Solid State and in Aqueous Solution. Drug Dev. Ind. Pharm. 1998, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Berber, M.R.; Hafez, I.H.; Mori, T.; Tanaka, M. Target Delivery and Controlled Release of the Chemopreventive Drug Sulindac by Using an Advanced Layered Double Hydroxide Nanomatrix Formulation System. J. Mater. Sci. Mater. Med. 2012, 23, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Dadej, A.; Geszke-Moritz, M.; Moritz, M.; Jelińska, A. Wpływ stopnia modyfikacji mezoporowatej krzemionki SBA-15 na proces adsorpcji sulindaku. Przemysł Chem. 2019, T. 98, nr 7. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. APTES-Modified Mesoporous Silicas as the Carriers for Poorly Water-Soluble Drug. Modeling of Diflunisal Adsorption and Release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Baranowski, M.; Woźniak-Braszak, A.; Jurga, K. High Homogeneity B(1) 30.2 MHz Nuclear Magnetic Resonance Probe for off-Resonance Relaxation Times Measurements. J. Magn. Reson. 2011, 208, 163–166. [Google Scholar] [CrossRef]
- Czechowski, T.; Baranowski, M.; Woźniak-Braszak, A.; Jurga, K.; Jurga, J.; Kędzia, P. The Instrument Set for Generating Fast Adiabatic Passage. Appl. Magn. Reson. 2012, 43, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Bilski, P.; Drużbicki, K.; Jenczyk, J.; Mielcarek, J.; Wąsicki, J. Molecular and Vibrational Dynamics in the Cholesterol-Lowering Agent Lovastatin: Solid-State NMR, Inelastic Neutron Scattering, and Periodic DFT Study. J. Phys. Chem. B 2017, 121, 2776–2787. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In Vitro Dissolution Profile Comparison--Statistics and Analysis of the Similarity Factor, F2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Tros de Ilarduya, M.C.; Martín, C.; Goñi, M.M.; Martínez-Ohárriz, M.C. Polymorphism of Sulindac: Isolation and Characterization of a New Polymorph and Three New Solvates. J. Pharm. Sci. 1997, 86, 248–251. [Google Scholar] [CrossRef]
- Samadi-Maybodi, A.; Sedighi-Pashaki, E. Comprehensive Study of Loading and Release of Sodium Valproate Drug Molecule from Functionalized SBA-15 with Aminopropyl Groups through Co-Condensation Modification Method. Mater. Chem. Phys. 2021, 257, 123622. [Google Scholar] [CrossRef]
- Guerra, R.B.; Gálico, D.A.; Holanda, B.B.C.; Bannach, G. Solid-State Thermal and Spectroscopic Studies of the Anti-Inflammatory Drug Sulindac Using UV–Vis, MIR, NIR, DSC, Simultaneous TG–DSC, and the Coupled Techniques TG-EGA-MIR and DSC–Optical Microscopy. J. Anal. Calorim. 2016, 123, 2523–2530. [Google Scholar] [CrossRef] [Green Version]
- Thahir, R.; Wahab, A.; La Nafie, N.; Raya, I. Synthesis of Mesoporous Silica SBA-15 through Surfactant Set-Up and Hydrothermal Process. Rasayan J. Chem. 2019, 12, 1117–1126. [Google Scholar] [CrossRef]
- Sayed, E.; Karavasili, C.; Ruparelia, K.; Haj-Ahmad, R.; Charalambopoulou, G.; Steriotis, T.; Giasafaki, D.; Cox, P.; Singh, N.; Giassafaki, L.-P.N.; et al. Electrosprayed Mesoporous Particles for Improved Aqueous Solubility of a Poorly Water Soluble Anticancer Agent: In Vitro and Ex Vivo Evaluation. J. Control. Release 2018, 278, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Letchmanan, K.; Shen, S.-C.; Ng, W.K.; Tan, R.B.H. Dissolution and Physicochemical Stability Enhancement of Artemisinin and Mefloquine Co-Formulation via Nano-Confinement with Mesoporous SBA-15. Colloids Surf. B Biointerfaces 2017, 155, 560–568. [Google Scholar] [CrossRef]
- Castaldo, R.; de Luna, M.S.; Siviello, C.; Gentile, G.; Lavorgna, M.; Amendola, E.; Cocca, M. On the Acid-Responsive Release of Benzotriazole from Engineered Mesoporous Silica Nanoparticles for Corrosion Protection of Metal Surfaces. J. Cult. Herit. 2020, 44, 317–324. [Google Scholar] [CrossRef]
- Abd-Elrahman, A.A.; El Nabarawi, M.A.; Hassan, D.H.; Taha, A.A. Ketoprofen Mesoporous Silica Nanoparticles SBA-15 Hard Gelatin Capsules: Preparation and in Vitro/in Vivo Characterization. Drug Deliv. 2016, 23, 3387–3398. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Hamidi, M. Dissolution Enhancement of a Model Poorly Water-Soluble Drug, Atorvastatin, with Ordered Mesoporous Silica: Comparison of MSF with SBA-15 as Drug Carriers. Expert Opin. Drug Deliv. 2016, 13, 171–181. [Google Scholar] [CrossRef]
- Maryam Hafezian, S.; Biparva, P.; Bekhradnia, A.; Naser Azizi, S. Amine and Thiol Functionalization of SBA-15 Nanoparticles for Highly Efficient Adsorption of Sulforaphane. Adv. Powder Technol. 2021, 32, 779–790. [Google Scholar] [CrossRef]
- Woźniak-Braszak, A.; Knitter, M.; Markiewicz, E.; Ingram, W.F.; Spontak, R.J. Effect of Composition on the Molecular Dynamics of Biodegradable Isotactic Polypropylene/Thermoplastic Starch Blends. ACS Sustain. Chem. Eng. 2019, 7, 16050–16059. [Google Scholar] [CrossRef]
- Makrocka-Rydzyk, M.; Woźniak-Braszak, A.; Jurga, K.; Jurga, S. Local Motions in Poly(Ethylene-Co-Norbornene) Studied by (1)H NMR Relaxometry. Solid State Nucl. Magn. Reson. 2015, 71, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys. Rev. 1948, 73, 679–712. [Google Scholar] [CrossRef]
- Slichter, C.P. Principles of Magnetic Resonance, 3rd ed.; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 978-3-540-50157-2. [Google Scholar]
- Abragam, A. The Principles of Nuclear Magnetism; Clarendon Press: New York, NY, USA, 1961; ISBN 978-0-19-852014-6. [Google Scholar]
- Beckmann, P.A. Spectral Densities and Nuclear Spin Relaxation in Solids. Phys. Rep. 1988, 171, 85–128. [Google Scholar] [CrossRef] [Green Version]
- Dobrzyńska-Mizera, M.; Knitter, M.; Woźniak-Braszak, A.; Baranowski, M.; Sterzyński, T.; Di Lorenzo, M.L. Poly(l-Lactic Acid)/Pine Wood Bio-Based Composites. Materials 2020, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Holderna-Natkaniec, K.; Jurga, K.; Natkaniec, I.; Nowak, D.; Szyczewski, A. Molecular Dynamics of Ethisterone Studied by 1H NMR, IINS and Quantum Mechanical Calculations. Chem. Phys. 2005, 317, 178–187. [Google Scholar] [CrossRef]
- Holderna-Natkaniec, K.; Natkaniec, I.; Kasperkowiak, W.; Sciesinska, E.; Sciesinski, J.; Mikuli, E. The IINS, IR and DFT Studies of Hydrogen Bonds in 6-Furfuryl and 6-Benzylaminopurines. J. Mol. Struct. 2006, 790, 94–113. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cole, R.H. Dielectric Relaxation in Glycerol, Propylene Glycol, and N-Propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Pajzderska, A.; Drużbicki, K.; Bilski, P.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Wąsicki, J. Environmental Effects on the Molecular Mobility of Ranitidine Hydrochloride: Crystalline State versus Drug Loaded into the Silica Matrix. J. Phys. Chem. Part C Nanomater. Interfaces Hard Matter 2019, 123, 18364–18375. [Google Scholar] [CrossRef]
- Marchetti, A.; Yin, J.; Su, Y.; Kong, X. Solid-State NMR in the Field of Drug Delivery: State of the Art and New Perspectives. Magn. Reson. Lett. 2021, 100003. [Google Scholar] [CrossRef]
- Yazdanian, M.; Briggs, K.; Jankovsky, C.; Hawi, A. The “High Solubility” Definition of the Current FDA Guidance on Biopharmaceutical Classification System May Be Too Strict for Acidic Drugs. Pharm. Res. 2004, 21, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, G.; Brouwers, J.; Snoeys, J.; Augustijns, P.; Vanuytsel, T. Insight into the Colonic Disposition of Sulindac in Humans. J. Pharm. Sci. 2021, 110, 259–267. [Google Scholar] [CrossRef]
- Morales, V.; Idso, M.; Balabasquer, M.; Chmelka, B.; Garcia-Muñoz, R. Correlating Surface-Functionalization of Mesoporous Silica With Adsorption and Release of Pharmaceutical Guest Species. J. Phys. Chem. C 2016, 120, 16887–16898. [Google Scholar] [CrossRef]
- Mellaerts, R.; Aerts, C.A.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Martens, J.A. Enhanced Release of Itraconazole from Ordered Mesoporous SBA-15 Silica Materials. Chem. Commun. 2007, 13, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Santos, H.A.; Kumar, N.; Murzin, D.Y.; Salonen, J.; Laaksonen, T.; Peltonen, L.; Hirvonen, J.; Lehto, V.-P. Cytotoxicity Study of Ordered Mesoporous Silica MCM-41 and SBA-15 Microparticles on Caco-2 Cells. Eur. J. Pharm. Biopharm. 2010, 74, 483–494. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Li, Y. Caco-2 Cell Permeability Assays to Measure Drug Absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z. PH-Responsive Poly (Acrylic Acid)-Gated Mesoporous Silica and Its Application in Oral Colon Targeted Drug Delivery for Doxorubicin. Colloids Surf. B Biointerfaces 2017, 154, 287–296. [Google Scholar] [CrossRef]








| Sample | Hindered Rotation of CH3 Group | Jump of Hydrogen Atom in Hydrogen Bonds | |||
|---|---|---|---|---|---|
| τ0 (s) | Ea (kJ/mol) | τ0 (s) | Ea (kJ/mol) | β | |
| SUL | 1.2 × 10−12 | 12.2 | 4.2 × 10−13 | 4.3 | - |
| SBA-15-A0.05:SUL | 2.2 × 10−11 | 12.7 | 2.1 × 10−11 | 4.7 | 0.2 |
| SBA-15-A0.20:SUL | 1.2 × 10−11 | 12.9 | 1.9 × 10−11 | 4.5 | 0.2 |
| Hydrochloric Acidic Medium pH = 1.2 | Phosphate Buffer pH = 5.8 | Phosphate Buffer pH = 6.8 | Phosphate Buffer pH = 7.4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBA-15-A0.20:SUL | SBA-15-A0.05:SUL | SUL | SBA-15-A0.20:SUL | SBA-15-A0.05:SUL | SUL | SBA-15-A0.20:SUL | SBA-15-A0.05:SUL | SUL | SBA-15-A0.20:SUL | SBA-15-A0.05:SUL | SUL | |
| SBA-15-A0.20:SUL | - | 43.03 | 9.34 | - | 44.92 | 20.29 | - | 37.28 | 33.23 | - | 85.36 | 37.08 |
| SBA-15-A0.05:SUL | - | 5.22 | - | 14.38 | - | 45.91 | - | 37.81 | ||||
| SUL | - | - | - | - | ||||||||
| SBA-15-A0.20:SUL | SBA-15-A0.05:SUL | |||||
|---|---|---|---|---|---|---|
| Phosphate Buffer pH = 5.8 | Phosphate Buffer pH = 6.8 | Phosphate Buffer pH = 7.4 | Phosphate Buffer pH = 5.8 | Phosphate Buffer pH = 6.8 | Phosphate Buffer pH = 7.4 | |
| Hydrochloric acidic medium pH = 1.2 | 85.49657 | 91.37849 | 66.31096 | 87.36534 | 70.3508 | 55.22457 |
| Phosphate buffer pH = 5.8 | - | 92.9635 | 66.12648 | - | 64.39402 | 59.18928 |
| Phosphate buffer pH = 6.8 | - | 64.98873 | - | 46.85479 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadej, A.; Woźniak-Braszak, A.; Bilski, P.; Piotrowska-Kempisty, H.; Józkowiak, M.; Geszke-Moritz, M.; Moritz, M.; Dadej, D.; Jelińska, A. Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica. Pharmaceutics 2021, 13, 1693. https://doi.org/10.3390/pharmaceutics13101693
Dadej A, Woźniak-Braszak A, Bilski P, Piotrowska-Kempisty H, Józkowiak M, Geszke-Moritz M, Moritz M, Dadej D, Jelińska A. Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica. Pharmaceutics. 2021; 13(10):1693. https://doi.org/10.3390/pharmaceutics13101693
Chicago/Turabian StyleDadej, Adrianna, Aneta Woźniak-Braszak, Paweł Bilski, Hanna Piotrowska-Kempisty, Małgorzata Józkowiak, Małgorzata Geszke-Moritz, Michał Moritz, Daniela Dadej, and Anna Jelińska. 2021. "Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica" Pharmaceutics 13, no. 10: 1693. https://doi.org/10.3390/pharmaceutics13101693
APA StyleDadej, A., Woźniak-Braszak, A., Bilski, P., Piotrowska-Kempisty, H., Józkowiak, M., Geszke-Moritz, M., Moritz, M., Dadej, D., & Jelińska, A. (2021). Modification of the Release of Poorly Soluble Sulindac with the APTES-Modified SBA-15 Mesoporous Silica. Pharmaceutics, 13(10), 1693. https://doi.org/10.3390/pharmaceutics13101693