Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy
Abstract
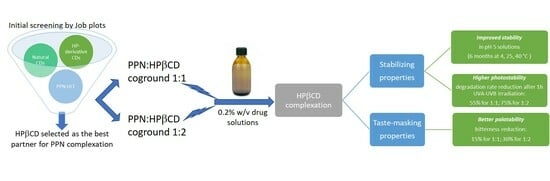
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Continuous Variation Method
2.3. Preparation of Drug-CD Solid Systems
2.4. Differential Scanning Calorimetry (DSC)
2.5. X-ray Powder Diffractometry (XRPD)
2.6. Chemical and Physical Stability Studies during Storage
2.7. Photo Stability Studies
2.8. Electronic Tongue Analysis
3. Results
3.1. Selection of the Most Suitable CD for PPN Complexation
3.2. Preparation and Characterization of Drug-HPβCD Solid Systems
3.3. Photo Stability Studies
3.4. Storage Stability Studies
3.5. Palatability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gore, R.; Chugh, P.K.; Tripathi, C.D.; Lhamo, Y.; Gautam, S. Pediatric Off-Label and Unlicensed Drug Use and Its Implications. Curr. Clin. Pharmacol. 2017, 12, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.C.; Garbe, M.C.; Lees, J.; Aziz, N.; Chaaban, H.; Miller, J.L.; Johnson, P.; de Leon, S. Off-Label medication use in children, more common than we think: A systematic review of the literature. J. Okla. State Med. Assoc. 2018, 111, 776–783. [Google Scholar] [PubMed]
- Moulis, F.; Durrieu, G.; Lapeyre-Mestre, M. Off-label and unlicensed drug use in children population. Therapie 2018, 73, 135–149. [Google Scholar] [CrossRef]
- Bjerknes, K.; Bøyum, S.; Kristensen, S.; Brustugun, J.; Wang, S. Manipulating tablets and capsules given to hospitalised children in Norway is common practice. Acta Paediatr. 2017, 106, 503–508. [Google Scholar] [CrossRef]
- Zahn Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of medicinal products for oral administration to paediatric patients at a German university hospital: An observational study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef]
- Tuleu, C.; Breitkreutz, J. Educational paper: Formulation-related issues in pediatric clinical pharmacology. Eur. J. Pediatr. 2013, 172, 717–720. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.; Currie, A.E.; Turner, M.A.; Field, D.J.; Mulla, H.; Pandya, H.C. Toxic additives in medication for preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F236–F240. [Google Scholar] [CrossRef]
- Belayneh, A.; Tadese, E.; Molla, F. Safety and biopharmaceutical challenges of excipients in off-label pediatric formulations. Int. J. Gen. Med. 2020, 13, 1051–1066. [Google Scholar] [CrossRef]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Zupanets, K.O.; Shebeko, S.K.; Ratushna, K.L.; Katilov, O.V. Cumulative Risks of Excipients in Pediatric Phytomucolytic Syrups. The Implications for Pharmacy Practice. Sci. Pharm. 2021, 89, 32. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Pharmaceutical Development of Medicines for Paediatric Use; EMA: London, UK, 2013; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 2 May 2023).
- Turner, M.A.; Duncan, J.C.; Shah, U.; Metsvaht, T.; Varendi, H.; Nellis, G.; Lutsar, I.; Yakkundi, S.; McElnay, J.C.; Jandya, H.; et al. Risk assessment of neonatal excipient exposure: Lessons from food safety and other areas. Adv. Drug Deliv. Rev. 2014, 73, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Nellis, G.; Metsvaht, T.; Varendi, H.; Toompere, K.; Lass, J.; Mesek, I.; Nunn, A.J.; Turner, M.A.; Lutsar, I. Potentially harmful excipients in neonatal medicines: A pan-European observational study. Arch Dis Child 2015, 100, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Lass, J.; Naelapää, K.; Shah, U.; Käär, R.; Varendi, H.; Turner, M.A.; Lutsar, I. Hospitalised neonates in Estonia commonly receive potentially harmful excipients. BMC Pediatr. 2012, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Questions and Answers on Propylene Glycol Used as an Excipient in Medicinal Products for Human Use; EMA: London, UK, 2017; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-propylene-glycol-used-excipient-medicinal-products-human-use_en.pdf (accessed on 2 May 2023).
- Zuccotti, G.V.; Fabiano, V. Safety issues with ethanol as an excipient in drugs intended for pediatric use. Expert Opin. Drug Saf. 2011, 10, 499–502. [Google Scholar] [CrossRef]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef]
- Saito, J.; Agrawal, A.; Patravale, V.; Pandya, A.; Orubu, S.; Zhao, M.; Andrews, G.P.; Petit-Turcotte, C.; Landry, H.; Croker, A.; et al. The Current States, Challenges, Ongoing Efforts, and Future Perspectives of Pharmaceutical Excipients in Pediatric Patients in Each Country and Region. Children 2022, 9, 453. [Google Scholar] [CrossRef]
- Ivanovska, V.; Rademaker, C.M.A.; Van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef]
- EMA (European Medicines Agency). Pre-authorisation of Medicines for Human USE. Reflection Paper: Formulations of Choice of the Paediatric Population. 28 July 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 24 June 2023).
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef]
- Great Ormond Street Hospital (GOSH) for Children. NHS Foundation Trust. Propranolol. January 2018. Available online: https://www.gosh.nhs.uk/conditions-and-treatments/medicines-information/propranolol/ (accessed on 24 June 2023).
- Tan, X.; Guo, S.; Wang, C. Propranolol in the Treatment of Infantile Hemangiomas. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1155–1163. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 62882, Propranolol Hydrochloride. 2023. In Remington’s Pharmaceutical Sciences, 15th ed.; Hoover, J.E., Osol, A., Eds.; Mack Publishing Co.: Easton, PA, USA, 1975; p. 835. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Propranolol-Hydrochloride (accessed on 2 May 2023).
- Martindale, W. The Extra Pharmacopoeia, 31st ed.; Reynolds, J.E.F., Ed.; Royal Pharmaceutical Society: London, UK, 1996; pp. 933–936. [Google Scholar]
- Giorgi, S. Codice di Galenica Clinica SIFO (Società Italiana di Farmacie Ospedaliera); Pensiero Scientifico Editore: Roma, Italy, 2010. [Google Scholar]
- Chaudhary, V.B.; Patel, J.K. Cyclodextrin inclusion complex to enhance solubility of poorly water soluble drugs: A review. Int. J. Pharm. Sci. Res. 2013, 4, 68–76. [Google Scholar]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Abou-taleb, A.E.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Taguchi, K.; Anraku, M.; Hirayama, F.; Uekama, K.; Otagiri, M. Evaluation of carboxymethyl-β-cyclodextrin with acid function: Improvement of chemical stability, oral bioavailability and bitter taste of famotidine. Int. J. Pharm. 2010, 397, 1–8. [Google Scholar] [CrossRef]
- Stojanov, M.; Wimmer, R.; Larsen, K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011, 100, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; EL-Massik, M.A.; Abdallah, O.Y.; Abdelkader, H. Reduction of Bitterness and Enhancing Palatability of Cetirizine Oral Liquid Dosage Forms by Cyclodextrins. J. Pharm. Drug Dev. 2014, 2, 102. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Kumar, D.; Singh, G. Formulation Development and Evaluation of Fast Disintegrating Tablet of Cetirizine Hydrochloride: A Novel Drug Delivery for Pediatrics and Geriatrics. J. Pharm. 2014, 2014, 808167. [Google Scholar] [CrossRef]
- Al Khazinddar, A.M.; Al Hameed, A.F.; Ajabnoor, A.M.A.; Ba-mansour, H.A.; Badr_Eldin Ali, S.M.; Salah Labib, G. Cetirizine fast dissolving tablets for dysphagic and pediatric patients. Pharm. Anal. Acta 2015, 6, 106. [Google Scholar] [CrossRef]
- Chay, S.K.; Keating, A.V.; James, C.; Aliev, A.E.; Haider, S.; Craig, D.Q.M. Evaluation of the taste-masking effects of (2-hydroxypropyl)-β-cyclodextrin on ranitidine hydrochloride; a combined biosensor, spectroscopic and molecular modelling assessment. RSC Adv. 2018, 8, 3564–3573. [Google Scholar] [CrossRef]
- Malaquias, L.F.B.; Sá-Barreto, L.C.L.; Freire, D.O.; Silva, I.C.R.; Karan, K.; Durig, T.; Lima, E.M.; Marreto, R.N.; Gelfuso, G.M.; Gratieri, T.; et al. Taste masking and rheology improvement of drug complexed with β-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohydr. Polym. 2018, 185, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Tokumura, T.; Tomono, K.; Suzuki, T.; Ueda, H.; Nagai, T.; Nagaoka, M.; Nakane, R.; Machida, Y. Effect of β-cyclodextrin on the degradation rate of amoxicillin in acidic solution. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2010, 130, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Garnero, C.; Aiassa, V.; Longhi, M. Sulfamethoxazole:hydroxypropyl-b-cyclodextrin complex: Preparation and characterization. J. Pharm. Biomed. Anal. 2012, 63, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Pomponio, R.; Gotti, R.; Fiori, J.; Cavrini, V.; Mura, P.; Cirri, M.; Maestrelli, F. Photostability studies on nicardipine-cyclodextrin complexes by capillary electrophoresis. J. Pharm. Biomed. Anal. 2004, 35, 267–725. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Li, S.; Yuan, L.; Zhang, B.; Zhou, W.; Wangab, X.; Baiab, D. Photostability and antioxidant activity studies on the inclusion complexes of trans-polydatin with β-cyclodextrin and derivatives. RSC Adv. 2018, 8, 25941–25948. [Google Scholar] [CrossRef] [PubMed]
- Ansolin, T.L.; Bariccatti, R.A.; da Rosa, M.F.; Lindino, C.A. The effect of β-cyclodextrin in the photochemical stability of propranolol hydrochloride in aqueous solution. Acta Sci. Technol. 2014, 36, 337–340. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products (CHMP). Cyclodextrins Used as Excipients; Report Published in Support of the “Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use” (EMA/CHMP/333892/2013); European Medicines Agency: London, UK, 2017. [Google Scholar]
- Cilurzo, F.; Cupone, I.E.; Minghetti, P.; Buratti, S.; Selmin, F.; Gennari, C.G.; Montanari, L. Nicotine fast dissolving films made of maltodextrins: A feasibility study. AAPS Pharmscitech 2010, 11, 1511–1517. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.G.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Borgognone, M.G.; Bussi, J.; Hough, G. Principal component analysis in sensory analysis: Covariance or correlation matrix? Food Qual. Prefer. 2001, 12, 323–326. [Google Scholar] [CrossRef]
- Connors, K.A. Binding Constants: The Measurement of Molecular Complex Stability; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Hirose, K.J. A Practical Guide for the Determination of Binding Constants. J. Incl. Phenom. Macrocycl. Chem. 2001, 39, 193–209. [Google Scholar] [CrossRef]
- Castronuovo, G.; Niccoli, M. Thermodynamics of inclusion complexes of natural and modified cyclodextrins with propranolol in aqueous solution at 298 K. Bioorg. Med. Chem. 2006, 14, 3883–3887. [Google Scholar] [CrossRef]
- Veiga, M.D.; Merino, M.; Cirri, M.; Maestrelli, F.; Mura, P. Comparative Study on Triclosan Interactions in Solution and in the Solid State with Natural and Chemically Modified Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 77–83. [Google Scholar] [CrossRef]
- Maestrelli, F.; Cecchi, M.; Cirri, M.; Capasso, G.; Mennini, N.; Mura, P. Comparative study of oxaprozin complexation with natural and chemically-modified cyclodextrins in solution and in the solid state. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 17–25. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Nerli, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Mennini, N. Improvement of Butamben Anesthetic Efficacy by the Development of Deformable Liposomes Bearing the Drug as Cyclodextrin Complex. Pharmaceutics 2021, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Cyclodextrins in Parenteral Formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Mura, P.A. Grinding as solvent-free green chemistry approach for cyclodextrin inclusion complex preparation in the solid state. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, G.; Mura, P.; Faucci, M.T.; Sorrenti, M.; Setti, M. Interaction of naproxen with noncrystalline acetyl beta- and acetyl gamma-cyclodextrins in the solid and liquid state. Eur. J. Pharm. Sci. 2002, 15, 21–29. [Google Scholar] [CrossRef]
- Mennini, N.; Bragagni, M.; Maestrelli, F.; Mura, P. Physico-chemical characterization in solution and in the solid state of clonazepam complexes with native and chemically-modified cyclodextrins. J. Pharm. Biomed. Anal. 2014, 89, 142–149. [Google Scholar] [CrossRef]
- Loftsson, T.; Ólafsdóttir, B.J. Cyclodextrin-accelerated degradation of β-lactam antibiotics in aqueous solutions. Int. J. Pharm. 1991, 67, R5–R7. [Google Scholar] [CrossRef]
- Popielec, A.; Fenyvesi, É.; Yannakopoulou, K.; Loftsson, T. Effect of cyclodextrins on the degradation rate of benzylpenicillin. Pharmazie 2016, 71, 68–75. [Google Scholar] [PubMed]
- European Medicines Agency (EMA). ICH Topic Q 1 E Evaluation of Stability Data, Note for Guidance on Evaluation of Stability Data; CPMP/ICH/420/02. EMA: London, UK, 2003. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-e-evaluation-stability-data-step-5_en.pdf (accessed on 23 August 2023).
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Combined Approach of Cyclodextrin Complexationand Nanostructured Lipid Carriers for the Development of a Pediatric Liquid Oral Dosage Form of Hydrochlorothiazide. Pharmaceutics 2018, 1, 287. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, A.H.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef]
- Yoshida, M.; Haraguchi, T.; Uchida, T. Bitterness evaluation of acidic pharmaceutical substances (NSAIDs) using a taste sensor. Chem. Pharm. Bull. 2014, 62, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wu, F.; Singh, V.; Guo, T.; Ren, X.; Yin, X.; Shao, Q.; York, P.; Patterson, L.H.; Zhang, J. Host-guest kinetic interactions between HP-β-cyclodextrin and drugs for prediction of bitter taste masking. J. Pharm. Biomed. Anal. 2017, 140, 232–238. [Google Scholar] [CrossRef]







| Components | Solution A | Solution B | Solution C |
|---|---|---|---|
| PPN | 0.2 g | 1.0 g | 0.1 g |
| Sucrose | 25 g | - | - |
| Citric acid anhydrous | 0.64 g | - | - |
| Na citrate dihydrate | 0.20 g | - | - |
| Raspberry flavour | 0.1 mL | 0.05 mL | 0.05 mL |
| Citric acid monohydrate | - | 0.60 g | 0.60 g |
| Simple syrup FU | - | q.b. to 100 mL | q.b. to 100 mL |
| Water for injections | q.b. to 100 mL | q.b. to solubilize PPN | q.b. to solubilize PPN |
| Sample | Tm (°C) | ΔHfus (J/g) | %RDC |
|---|---|---|---|
| PPN | 163.92 | 121.91 | 100.0 |
| PPN ground 15′ at 24 Hz | 162.22 | 112.59 | 92.30 |
| PPN ground 30 at 24 Hz | 162.81 | 110.27 | 90.45 |
| PPN-HPβCD PM 1:1 | 164.20 | 90.20 | 73.98 |
| PPN-HPβCD PM 1:2 | 163.80 | 85.52 | 70.15 |
| PPN-HPβCD GR 1:1 | - | - | - |
| PPN-HPβCD GR 1:2 | - | - | - |
| Solution Sample | K0–1 h (%/min) | % Stabilization | K0–5 h (%/min) | % Stabilization |
|---|---|---|---|---|
| PPN | −0.2014 | −0.0891 | ||
| PPN: HPβCD 1:1 PM | −0.1517 | 24.7 | −0.0626 | 29.7 |
| PPN: HPβCD 1:2 PM | −0.0956 | 52.5 | −0.0402 | 54.9 |
| PPN: HPβCD 1:1 GR | −0.0775 | 61.5 | −0.0594 | 34.4 |
| PPN: HPβCD 1:2 GR | −0.0541 | 73.1 | −0.0398 | 55.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirri, M.; Mura, P.; Benedetti, S.; Buratti, S. Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics 2023, 15, 2217. https://doi.org/10.3390/pharmaceutics15092217
Cirri M, Mura P, Benedetti S, Buratti S. Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics. 2023; 15(9):2217. https://doi.org/10.3390/pharmaceutics15092217
Chicago/Turabian StyleCirri, Marzia, Paola Mura, Simona Benedetti, and Susanna Buratti. 2023. "Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy" Pharmaceutics 15, no. 9: 2217. https://doi.org/10.3390/pharmaceutics15092217
APA StyleCirri, M., Mura, P., Benedetti, S., & Buratti, S. (2023). Development of a Hydroxypropyl-β-Cyclodextrin-Based Liquid Formulation for the Oral Administration of Propranolol in Pediatric Therapy. Pharmaceutics, 15(9), 2217. https://doi.org/10.3390/pharmaceutics15092217