Influence of Ellagitannins Extracted by Pomegranate Fruit on Disulfide Isomerase PDIA3 Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
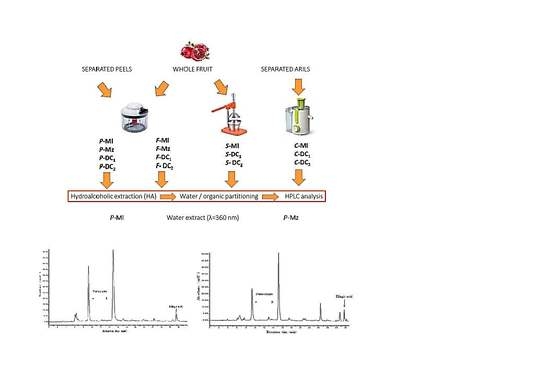
2.2. Samples Preparation
2.3. Extraction Methods
2.3.1. Whole Fruit or Peels Extraction with Ethanol and Acidified Water
2.3.2. Juice Extraction with Ethanol
2.3.3. Partitioning in Ethyl Acetate
2.4. HPLC-DAD Analyses
2.5. PDIA3 Purification and Disulfide Reductase Activity Determination
2.5.1. Protein Expression and Purification
2.5.2. Determination of Protein Disulfide Reductase Activity
2.6. Statistical Analysis
3. Results
3.1. Polyphenols Extraction
3.2. Quali-Quantitative Analysis of the Polyphenolic Fraction
3.3. Pomegranate Extract Effects on Disulfide Redox Activity of the PDIA3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: From diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Jalal, H.; Pal, M.A.; Ahmad, S.R.; Rather, M.; Andrabi, M.; Hamdani, S. Physico-chemical and functional properties of pomegranate peel and seed powder. J. Pharm. Innov. 2018, 7, 1127–1131. [Google Scholar]
- Khatib, M.; Innocenti, M.; Giuliani, C.; Al-Tamimi, A.; Romani, A.; Mulinacci, N. Mesocarp and exocarp of Laffan and wonderful pomegranate varieties: By-products as a source of ellagitannins. Int. J. Food Nutr. Sci. 2017, 4, 1–7. [Google Scholar]
- Bellone, J.A.; Murray, J.R.; Jorge, P.; Fogel, T.G.; Kim, M.; Wallace, D.R.; Hartman, R.E. Pomegranate supplementation improves cognitive and functional recovery following ischemic stroke: A randomized trial. Nutr. Neurosci. 2018, 1–6. [Google Scholar] [CrossRef]
- Tortora, K.; Femia, A.P.; Romagnoli, A.; Sineo, I.; Khatib, M.; Mulinacci, N.; Giovannelli, L.; Caderni, G. Pomegranate by-products in colorectal cancer chemoprevention: Effects in Apc-mutated Pirc rats and mechanistic studies in vitro and ex vivo. Mol. Nutr. Food Res. 2018, 62, 1700401. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Zirak, M.R.; Sahebkar, A. Ellagic acid: A logical lead for drug development? Curr. Pharm. Des. 2017, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A pharmacological update of ellagic acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Turrini, F.; Villa, C.; Lacapra, C.; Zunin, P.; Parodi, B. Green extraction from pomegranate marcs for the production of functional foods and cosmetics. Pharmaceuticals (Basel) 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Qian, W.; Su, Y.; Zhang, Y.; Yao, N.; Gu, N.; Zhang, X.; Yin, H. Secretome analysis of rat osteoblasts during icariin treatment induced osteogenesis. Mol. Med. Rep. 2018, 17, 6515–6525. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Nam, M.; Lee, D.; Park, J.W.; Kang, B.; Lee, D.S.; Lee, H.S.; Kwon, O.S. Silibinin ameliorates O-GlcNacylation and inflammation in a mouse model of nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2165. [Google Scholar] [CrossRef]
- Trnková, L.; Ricci, D.; Grillo, C.; Colotti, G.; Altieri, F. Green tea catechins can bind and modify ERp57/PDIA3 activity. Biochim. Biophys. Acta 2013, 1830, 2671–2682. [Google Scholar] [CrossRef]
- Grillo, C.; Chichiarelli, S.; Gaucci, E.; Altieri, F.; Turano, C.; Cervoni, L. The binding of silibinin to ERp57. Chem. Biol. Interact. 2014, 213, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Giamogante, F.; Marrocco, I.; Romaniello, D.; Eufemi, M.; Chichiarelli, S.; Altieri, F. Comparative analysis of the interaction between different flavonoids and PDIA3. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Giamogante, F.; Marrocco, I.; Cervoni, L.; Eufemi, M.; Chichiarelli, S.; Altieri, F. Punicalagin, an active pomegranate component, is a new inhibitor of PDIA3 reductase activity. Biochimie 2018, 147, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Turano, C.; Gaucci, E.; Grillo, C.; Chichiarelli, S. ERp57/GRP58: A protein with multiple functions. Cell. Mol. Biol. Lett. 2011, 16, 539–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetz, C.; Russelakis-Carneiro, M.; Wälchli, S.; Carboni, S.; Vial-Knecht, E.; Maundrell, K.; Castilla, J.; Soto, C. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J. Neurosci. 2005, 25, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Erickson, R.R.; Dunning, L.M.; Olson, D.A.; Cohen, S.J.; Davis, A.T.; Wood, W.G.; Kratzke, R.A.; Holtzman, J.L. In cerebrospinal fluid ER chaperones ERp57 and calreticulin bind β-amyloid. Biochem. Biophys. Res. Commun. 2005, 332, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kim-Han, J.S.; O’Malley, K.L. Cell stress induced by the parkinsonian mimetic, 6-hydroxydopamine, is concurrent with oxidation of the chaperone, ERp57, and aggresome formation. Antioxid. Redox Signal. 2007, 9, 2255–2264. [Google Scholar] [CrossRef]
- Laurindo, F.R.; Pescatore, L.A.; de Castro Fernandes, D. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radic. Biol. Med. 2012, 52, 1954–1969. [Google Scholar] [CrossRef]
- Maattanen, P.; Kozlov, G.; Gehring, K.; Thomas, D.Y. ERp57 and PDI: Multifunctional protein disulfide isomerases with similar domain architectures but differing substrate–partner associations. Biochem. Cell Biol. 2006, 84, 881–889. [Google Scholar] [CrossRef]
- Holbrook, L.M.; Sasikumar, P.; Stanley, R.G.; Simmonds, A.D.; Bicknell, A.B.; Gibbins, J.M. The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function. J. Thromb. Haemost. 2012, 10, 278–288. [Google Scholar] [CrossRef]
- Wu, Y.; Ahmad, S.S.; Zhou, J.; Wang, L.; Cully, M.P.; Essex, D.W. The disulfide isomerase ERp57 mediates platelet aggregation, hemostasis, and thrombosis. Blood 2012, 119, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Bendapudi, P.; Sharda, A.; Chen, V.; Bellido-Martin, L.; Jasuja, R.; Furie, B.C.; Flaumenhaft, R.; Furie, B. Extracellular thiol isomerases and their role in thrombus formation. Antioxid. Redox Signal. 2016, 24, 1–15. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Ren, J.; Fang, J.; Ke, H.; Gong, W.; Wang, C.C. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxid. Redox Signal. 2013, 19, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Wang, L.; Rauova, L.; Hayes, V.M.; Poncz, M.; Essex, D.W. The disulfide isomerase ER p57 is required for fibrin deposition in vivo. J. Thromb. Haemost. 2014, 12, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Shan, L.; Guo, L.; Chu, I.K.; Li, G.; Quan, Q.; Zhao, Y.; Chong, C.M.; Zhang, Z.; Yu, P.; et al. Novel anti-thrombotic agent for modulation of protein disulfide isomerase family member ERp57 for prophylactic therapy. Sci. Rep. 2015, 5, 10353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. aerial parts: Extraction procedures, secondary metabolites and color analysis. Molecules 2018, 23, 1962–1973. [Google Scholar] [CrossRef]
- Coppari, S.; Altieri, F.; Ferraro, A.; Chichiarelli, S.; Eufemi, M.; Turano, C. Nuclear localization and DNA interaction of protein disulfide isomerase ERp57 in mammalian cells. J. Cell. Biochem. 2002, 85, 325–333. [Google Scholar] [CrossRef]
- Grillo, C.; D’ambrosio, C.; Scaloni, A.; Maceroni, M.; Merluzzi, S.; Turano, C.; Altieri, F. Cooperative activity of Ref-1/APE and ERp57 in reductive activation of transcription factors. Free Radic. Biol. Med. 2006, 41, 1113–1123. [Google Scholar] [CrossRef]
- Grillo, C.; D’Ambrosio, C.; Consalvi, V.; Chiaraluce, R.; Scaloni, A.; Maceroni, M.; Eufemi, M.; Altieri, F. DNA-binding activity of the ERp57 C-terminal domain is related to a redox-dependent conformational change. J. Biol. Chem. 2007, 282, 10299–10310. [Google Scholar] [CrossRef]
- Raturi, A.; Mutus, B. Characterization of redox state and reductase activity of protein disulfide isomerase under different redox environments using a sensitive fluorescent assay. Free Radic. Biol. Med. 2007, 43, 62–70. [Google Scholar] [CrossRef]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]



| Sample | Hydroalcoholic (HA) | Water (W) | Organic | |||
|---|---|---|---|---|---|---|
| Punicalagin | Ellagic Acid | Punicalagin | Ellagic Acid | Punicalagin | Ellagic Acid | |
| F-DC1 | - | - | 5.13 ± 0.11 | 0.07 ± 0.02 | - | - |
| P-DC1 | - | - | 25.9 ± 0.02 | 0.76 ± 0.07 | - | - |
| C-DC1 | - | - | 0.05 ± 0.02 | 0.006 ± 0.003 | - | - |
| S-DC1 | - | - | 1.26 ± 0.10 | 0.017 ± 0.003 | - | - |
| F-DC2 | - | - | 6.53 ± 0.09 | 0.22 ± 0.09 | - | - |
| P-DC2 | - | - | 16.2 ± 0.01 | 0.80 ± 0.04 | - | - |
| C-DC2 | - | - | 0.06 ± 0.04 | 0.007 ± 0.004 | - | - |
| S-DC2 | - | - | 0.72 ± 0.05 | 0.008 ± 0.004 | - | - |
| F-Mz | 11.2 ± 0.11 | 1.57 ± 0.02 | 10.4 ± 0.12 | 0.34 ± 0.08 | 36.7 ± 0.20 | 24.6 ± 0.13 |
| P-Mz | 25.7 ± 0.24 | 3.40 ± 0.19 | 24.9 ± 0.05 | 1.08 ± 0.13 | 35.1 ± 0.14 | 37.4 ± 0.05 |
| F-Ml | 19.1 ± 0.20 | 0.59 ± 0.03 | 15.8 ± 0.15 | 0.32 ± 0.07 | 54.8 ± 0.26 | 14.7 ± 0.09 |
| P-Ml | 32.2 ± 0.35 | 1.00 ± 0.25 | 30.3 ± 0.17 | 0.40 ± 0.08 | 73.5 ± 0.58 | 17.8 ± 0.06 |
| C-Ml | 0.06 ± 0.03 | 0.012 ± 0.003 | 0.05 ± 0.01 | 0.007 ± 0.002 | BLD * | 2.45 ± 0.50 |
| S-Ml | 0.85 ± 0.45 | 0.026 ± 0.007 | 0.79 ± 0.07 | 0.01 ± 0.009 | BLD * | 1.25 ± 0.30 |
| Sample | Hydroalcoholic (HA) | Water (W) | ||||||
|---|---|---|---|---|---|---|---|---|
| From Table 1 | Calculated | From Table 1 | Calculated | |||||
| Pun/extr mg/g | IC50 extr mg/L | IC50 Pun µg/L | IC50 Pun µM | Pun/extr mg/g | IC50 extr mg/L | IC50 Pun µg/L | IC50 Pun µM | |
| A | B | AxB | AxB/MW | A | B | AxB | AxB/MW | |
| F-Mz | 11.2 | 15.07 | 168.8 | 0.156 | 10.4 | 10.54 | 109.2 | 0.101 |
| P-Mz | 25.7 | 4.69 | 120.3 | 0.111 | 24.9 | 3.48 | 86.7 | 0.080 |
| F-Ml | 19.1 | 9.78 | 186.8 | 0.172 | 15.8 | 2.53 | 40.0 | 0.037 |
| P-Ml | 32.2 | 6.00 | 193.2 | 0.178 | 30.3 | 0.69 | 20.9 | 0.019 |
| F-DC1 | - | - | - | - | 5.13 | 5.87 | 30.2 | 0.028 |
| P-DC1 | - | - | - | - | 25.88 | 3.42 | 88.6 | 0.082 |
| F-DC2 | - | - | - | - | 6.53 | 3.4 | 22.2 | 0.020 |
| P-DC2 | - | - | - | - | 16.14 | 1.37 | 22.2 | 0.020 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altieri, F.; Cairone, F.; Giamogante, F.; Carradori, S.; Locatelli, M.; Chichiarelli, S.; Cesa, S. Influence of Ellagitannins Extracted by Pomegranate Fruit on Disulfide Isomerase PDIA3 Activity. Nutrients 2019, 11, 186. https://doi.org/10.3390/nu11010186
Altieri F, Cairone F, Giamogante F, Carradori S, Locatelli M, Chichiarelli S, Cesa S. Influence of Ellagitannins Extracted by Pomegranate Fruit on Disulfide Isomerase PDIA3 Activity. Nutrients. 2019; 11(1):186. https://doi.org/10.3390/nu11010186
Chicago/Turabian StyleAltieri, Fabio, Francesco Cairone, Flavia Giamogante, Simone Carradori, Marcello Locatelli, Silvia Chichiarelli, and Stefania Cesa. 2019. "Influence of Ellagitannins Extracted by Pomegranate Fruit on Disulfide Isomerase PDIA3 Activity" Nutrients 11, no. 1: 186. https://doi.org/10.3390/nu11010186
APA StyleAltieri, F., Cairone, F., Giamogante, F., Carradori, S., Locatelli, M., Chichiarelli, S., & Cesa, S. (2019). Influence of Ellagitannins Extracted by Pomegranate Fruit on Disulfide Isomerase PDIA3 Activity. Nutrients, 11(1), 186. https://doi.org/10.3390/nu11010186