Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats
Abstract
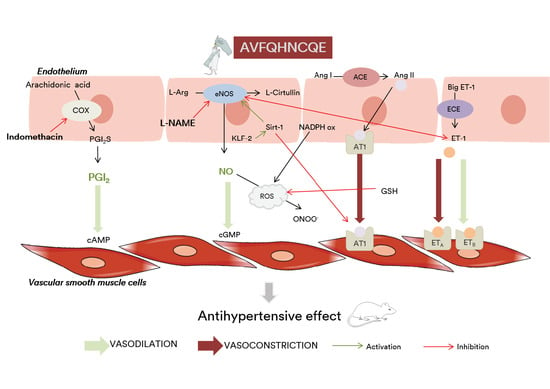
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Procedure in Animals
2.3. Determination of Plasma ACE Activity
2.4. Reduced Glutathione Assay
2.5. Measurement of Malondialdehyde Production
2.6. RNA Extraction and mRNA Quantification by Real-Time qPCR
2.7. Experiments in Aortic Rings
2.8. Statistical Analysis
3. Results
3.1. Effects of AVFQHNCQE on Blood Pressure in SHR Treated with L-NAME or Indomethacin
3.2. Effects of AVFQHNCQE on Plasma ACE Activity, Liver GSH Concentration, Malodialdehyde Production, and Endothelial-Related Gene Expression
3.3. Effects of AVFQHNCQE on Aortic Rings
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messerli, F.H.; Williams, B.; Ritz, E. Essential hypertension. Lancet 2007, 370, 591–603. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Ž. How to improve cardiovascular diseases prevention in Europe? Nutr. Metab. Cardiovasc. Dis. 2009, 19, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Antonakoudis, G.; Poulimenos, L.; Kifnidis, K.; Zouras, C.; Antonakoudis, H. Blood pressure control and cardiovascular risk reduction. Hippokratia 2007, 11, 114–119. [Google Scholar] [PubMed]
- Quirós, A.; del Mar Contreras, M.; Ramos, M.; Amigo, L.; Recio, I. Stability to gastrointestinal enzymes and structure–activity relationship of β-casein-peptides with antihypertensive properties. Peptides 2009, 30, 1848–1853. [Google Scholar] [CrossRef]
- Yamamoto, N. Antihypertensive peptides derived from food proteins. Biopolym. Pept. Sci. Sect. 1997, 43, 129–134. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Atlas, S.A. The renin-angiotensin aldosterone system: Pathophysiological role and pharmacologic inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, D.; Tanokura, M. Optimisation of hydrolysis conditions for the production of the angiotensin-I converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology. Food Chem. 2009, 114, 328–333. [Google Scholar] [CrossRef]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived ACE-inhibitory peptides IQW and LKP reduce blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2015, 13, 50–60. [Google Scholar] [CrossRef]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; Lopez-Fandino, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and anti hypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, A.; Nakamura, T.; Hirota, T.; Matsushita, A.; Asakura, M.; Ohki, K.; Kitakaze, M. The milk-derived peptides Val-Pro-Pro and Ile-Pro-Pro attenuate arterial dysfunction in L-NAME-treated rats. Hypertens. Res. 2014, 37, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Matoba, N.; Usui, H.; Fujita, H.; Yoshikawa, M. A novel anti-hypertensive peptide derived from ovalbumin induces nitric oxide-mediated vasorelaxation in an isolated SHR mesenteric artery. FEBS Lett. 1999, 452, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Durante, W.; Johnson, F.K.; Johnson, R.A. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Grape seed flavanols decrease blood pressure via Sirt-1 and confer a vasoprotective pattern in rats. J. Funct. Foods 2016, 24, 164–172. [Google Scholar] [CrossRef]
- Atkins, G.B.; Jain, M.K. Role of Krüppel-Like Transcription Factors in Endothelial Biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Pons, Z.; Margalef, M.; Arola-Arnal, A.; Muguerza, B. Regulation of Vascular Endothelial Genes by Dietary Flavonoids: Structure-Expression Relationship Studies and the Role of the Transcription Factor KLF-2. J. Nutr. Biochem. 2014, 26, 277–284. [Google Scholar] [CrossRef]
- Zarzuelo, M.J.; López-Sepúlveda, R.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Ungvary, Z.; Pérez-Vizcaíno, F.; Jiménez, R.; Duarte, J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: Implications for vascular aging. Biochem. Pharmacol. 2013, 85, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Zalba, G.; Beaumont, F.J.; José, G.S.; Fortuño, A.; María, A.; Etayo, J.C.; Díez, J.; Jose, G.S.; Fortun, A.; Dı, J. Vascular NADH/NADPH Oxidase Is Involved in Enhanced Superoxide Production in Spontaneously Hypertensive Rats. Hypertension 2000, 35, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Bravo, F.I.; Arola, L.; Muguerza, B. Procedure for Obtaining a Hydrolyzate Claw Chicken Leg with Antihypertensive Activity, and Peptides Obtained Hydrolyzate Containing. ES Patent ES2606954A1, 11 December 2017. [Google Scholar]
- Buñag, R.D.; Butterfield, J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension 1982, 4, 898–903. [Google Scholar] [CrossRef]
- Miguel, M.; Alonso, M.J.; Salaices, M.; Aleixandre, A.; López-Fandiño, R. Antihypertensive, ACE-inhibitory and vasodilator properties of an egg white hydrolysate: Effect of a simulated intestinal digestion. Food Chem. 2007, 104, 163–168. [Google Scholar] [CrossRef]
- Kamencic, H.; Lyon, A.; Paterson, P.G.; Juurlink, B.H.J. Monochlorobimane Fluorometric Method to Measure. Anal. Biochem. 2000, 37, 35–37. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, M.A.; Ruiz-Torres, A. Homeostasis between lipid peroxidation and antioxidant enzyme activities in healthy human aging. Mech. Ageing Dev. 1992, 66, 213–222. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011. [Google Scholar] [CrossRef] [PubMed]
- Yuliatmo, R.; Fitriyanto, N.A.; Bachruddin, Z.; Erwanto, Y. Increasing of angiotensin converting enzyme inhibitory derived from chicken leg. Int. Food Res. J. 2017, 24, 1799–1804. [Google Scholar]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Terashima, M.; Baba, T.; Ikemoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the Mechanisms of Action Based on the Pathophysiology of Hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. Endogenous nitric oxide: Physiology, pathology and clinical relevance. Eur. J. Clin. Investig. 1991, 21, 361–374. [Google Scholar] [CrossRef]
- Loscalzo, J.; Welch, G. Nitric oxide and its role in the cardiovascular system. Prog. Cardiovasc. Dis. 1995, 38, 87–104. [Google Scholar] [CrossRef]
- Kouguchi, T.; Zhang, Y.; Sato, M.; Takahata, Y.; Morimatsu, F. Vasoprotective Effect of Foods as Treatments: Chicken Collagen Hydrolysate. In Atherogenesis; Parthasarathy, S., Ed.; InTech: London, UK, 2012; Volume 26, pp. 557–570. [Google Scholar]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Wind, S.; Beuerlein, K.; Armitage, M.E.; Taye, A.; Kumar, A.H.S.; Janowitz, D.; Neff, C.; Shah, A.M.; Wingler, K.; Schmidt, H.H.H.W. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 2010, 56, 490–497. [Google Scholar] [CrossRef]
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Kim, G.R.; Seok, Y.M.; Lin, M.Q.; Ryu, Y.; Choi, S.Y.; Kee, H.J.; et al. Gallic Acid Reduces Blood Pressure and Attenuates Oxidative Stress and Cardiac Hypertrophy in Spontaneously Hypertensive Rats. Sci. Rep. 2017, 7, 15607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manso, M.A.; Miguel, M.; Even, J.; Hernández, R.; Aleixandre, A.; López-Fandiño, R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008, 109, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Shimizu, T.; Tsounapi, P.; Higashi, Y.; Martin, D.T.; Nakamura, K.; Honda, M.; Inoue, K.; Saito, M. Effect of silodosin, an alpha1A-adrenoceptor antagonist, on ventral prostatic hyperplasia in the spontaneously hypertensive rat. PLoS ONE 2015, 10, e0133798. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Sahar, A.; Arshad, M.S.; Rahman, U.U.; Imran, A.; Hussain, S. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int. J. Food Prop. 2017, 20, 2581–2593. [Google Scholar] [CrossRef]
- Suzuki, H.; Swei, A.; Zweifach, B.W.; Schmid-Schönbein, G.W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension 1995, 25, 1083–1089. [Google Scholar] [CrossRef]
- Negishi, H.; Njelekela, M.; Ikeda, K.; Sagara, M.; Noguchi, T.; Kuga, S.; Kanda, T.; Liu, L.; Nara, Y.; Tagami, M.; et al. Assessment of in vivo oxidative stress in hypertensive rats and hypertensive subjects in Tanzania, Africa. Hypertens. Res. 2000, 23, 285–289. [Google Scholar] [CrossRef]
- Masaki, T.; Sawamura, T. Endothelin and endothelial dysfunction. Proc. Jpn. Acad. Ser. B 2006, 82, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH Oxidases in Vascular Pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [Green Version]
- Maes, W.; Van Camp, J.; Vermeirssen, V.; Hemeryck, M.; Ketelslegers, J.M.; Schrezenmeir, J.; Van Oostveldt, P.; Huyghebaert, A. Influence of the lactokinin Ala-Leu-Pro-Met-His-Ile-Arg (ALPMHIR) on the release of endothelin-1 by endothelial cells. Regul. Pept. 2004, 118, 105–109. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; López-Díez, J.J.; Torregrosa, G.; Vallés, S.; Alborch, E.; Manzanares, P.; Salom, J.B. Lactoferricin B-derived peptides with inhibitory effects on ECE-dependent vasoconstriction. Peptides 2010, 31, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Matsuno, K.; Iwata, K.; Ibi, M.; Katsuyama, M.; Kakehi, T.; Sasaki, M.; Ikami, K.; Zhu, K.; Yabe-Nishimura, C. NADPH Oxidase Isoforms and Anti-hypertensive Effects of Atorvastatin Demonstrated in Two Animal Models. J. Pharmacol. Sci. 2009, 111, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, Z.; Arola, L. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2013, 6, 419–427. [Google Scholar]
- Mitchell, J.A.; Ali, F.; Bailey, L.; Moreno, L.; Harrington, L.S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008, 93, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Gryglewski, R.; Bunting, S.; Vane, J.R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976, 263, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamauchi, D.; Usui, H.; Zhao, H.; Yokoo, M.; Ohinata, K.; Iwai, M.; Horiuchi, M.; Yoshikawa, M. Hypotensive activity of novokinin, a potent analogue of ovokinin(2–7), is mediated by angiotensin AT2 receptor and prostaglandin IP receptor. Peptides 2008, 29, 412–418. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Sakaguchi, M.; Miyazaki, M. Significant target organs for hypertension and cardiac hypertrophy by angiotensin-converting enzyme inhibitors. Hypertens. Res. 2004, 27, 213–219. [Google Scholar] [CrossRef]
- Martin, M.; Deussen, A. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit. Rev. Food Sci. Nutr. 2017, 8398, 1–20. [Google Scholar] [CrossRef]
- Mine, Y.; Shahidi, F. Nutraceutical Proteins and Peptides in Health and Disease; CRC/Taylor and Francis: Boca Raton, FL, USA, 2006; ISBN 9781420028836. [Google Scholar]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef]
- Nurminen, M.L.; Sipola, M.; Kaarto, H.; Pihlanto-Leppälä, A.; Piilola, K.; Korpela, R.; Tossavainen, O.; Korhonen, H.; Vapaatalo, H. Alpha-lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sci. 2000, 66, 1535–1543. [Google Scholar] [CrossRef]
- Sipola, M.; Finckenberg, P.; Vapaatalo, H.; Pihlanto-Leppälä, A.; Korhonen, H.; Korpela, R.; Nurminen, M.-L. Alpha-lactorphin and beta-lactorphin improve arterial function in spontaneously hypertensive rats. Life Sci. 2002, 71, 1245–1253. [Google Scholar] [CrossRef]
- Costerousse, O.; Allegrini, J.; Clozel, J.P.; Ménard, J.; Alhenc-Gelas, F. Angiotensin I-converting enzyme inhibition but not angiotensin II suppression alters angiotensin I-converting enzyme gene expression in vessels and epithelia. J. Pharmacol. Exp. Ther. 1998, 284, 1180–1187. [Google Scholar] [PubMed]








| Rat Primers | Sequence (5′……3′) | Size of Amplicon | Efficiency | GenBank Accession No. |
|---|---|---|---|---|
| eNOS Fw | GGATTCTGGCAAGACCGATTAC | 159 | 2.06 (100.67%) | NM_021838.2 |
| eNOS Rv | GGTGAGGACTTGTCCAAACACT | |||
| Arg-1 Fw | GGCAGTGGCGTTGACCTTGT | 158 | 2.14 (105.64%) | NM_017134.3 |
| Arg-1 Rv | AGCAGCGTTGGCCTGGTTCT | |||
| Sirt Fw | TTGGCACCGATCCTCGAA | 217 | 2.02 (97.49%) | NM_001007684.1 |
| Sirt Rv | ACAGAAACCCCAGCTCCA | |||
| KLF2 Fw | GGACGCACACAGGTGAGAA | 186 | 2.18 (107.85%) | NM_001007684.1 |
| KLF2 Rv | ACATGTGTCGCTTCATGTGC | |||
| ET-1 Fw | TGATTCTCTTGCCTCTTCTTG | 110 | 3.08 (136.04%) | NM_012548.2 |
| ET-1 Rv | TATGGAATCTCCTGGCTCTC | |||
| NOX4 Fw | GTGTCTGCATGGTGGTGGTA | 150 | 2.24 (110.62%) | NM_053524.1 |
| NOX4 Rv | TCAACAAGCCACCCGAAACA | |||
| GADPH Fw | CCATGTTCGTCATGGGTGTG | 91 | 1.97 (98.93%) | NM_002046.4 |
| GADPH Rv | GGTGCTAAGCAGTTGGTGGTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Aragonès, G.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats. Nutrients 2019, 11, 225. https://doi.org/10.3390/nu11020225
Mas-Capdevila A, Iglesias-Carres L, Arola-Arnal A, Aragonès G, Aleixandre A, Bravo FI, Muguerza B. Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats. Nutrients. 2019; 11(2):225. https://doi.org/10.3390/nu11020225
Chicago/Turabian StyleMas-Capdevila, Anna, Lisard Iglesias-Carres, Anna Arola-Arnal, Gerard Aragonès, Amaya Aleixandre, Francisca I. Bravo, and Begoña Muguerza. 2019. "Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats" Nutrients 11, no. 2: 225. https://doi.org/10.3390/nu11020225
APA StyleMas-Capdevila, A., Iglesias-Carres, L., Arola-Arnal, A., Aragonès, G., Aleixandre, A., Bravo, F. I., & Muguerza, B. (2019). Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats. Nutrients, 11(2), 225. https://doi.org/10.3390/nu11020225