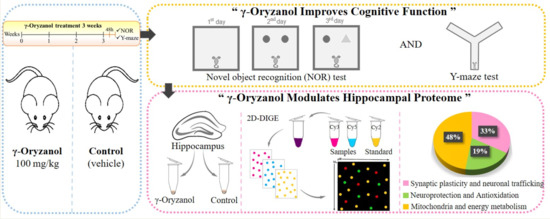
γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Dose Calculation
2.3. Chemicals and Treatment
2.4. Rotarod Test
2.5. Y-Maze
2.6. Novel Object Recognition (NOR)
2.7. Two-Dimensional Electrophoresis
2.8. Peptide Preparation
2.9. Liquid Chromatography–Mass Spectrometry (LC–MS) analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. γ-Oryzanol Improves Cognitive Function in Adult Mice
3.2. γ-Oryzanol Modulates Hippocampal Proteome
4. Discussion
4.1. Synaptic Plasticity and Neuronal Trafficking
4.2. Neuroprotection and Antioxidant Activity
4.3. Mitochondria and Energy Metabolism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Q.; Spiegelman, D.; van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White rice, brown rice, and risk of type 2 diabetes in us men and women. Arch. Intern. Med. 2010, 170, 961–969. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Upland rice and allelopathy. Commun. Soil Sci. Plant Anal. 2003, 34, 1311–1329. [Google Scholar] [CrossRef]
- Lin, Y.T.; Pao, C.C.; Wu, S.T.; Chang, C.Y. Effect of different germination conditions on antioxidative properties and bioactive compounds of germinated brown rice. BioMed Res. Int. 2015, 2015, 608761. [Google Scholar] [CrossRef]
- Heuberger, A.L.; Lewis, M.R.; Chen, M.H.; Brick, M.A.; Leach, J.E.; Ryan, E.P. Metabolomic and functional genomic analyses reveal varietal differences in bioactive compounds of cooked rice. PLoS ONE 2010, 5, e12915. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Therapeutic properties of rice constituents and derivatives (oryza sativa l.): A review update. Trends Food Sci. Technol. 2014, 40, 82–98. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, S.T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Kaneko, R.; Tsuchiya, T. New compound in rice bran and germ oils (abstract in English). Kogyo Kagaku Zasshi 1954, 57, 526–529. [Google Scholar] [CrossRef]
- Xu, Z.; Godber, J.S. Purification and identification of components of gamma-oryzanol in rice bran oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Correa, C.R.; Lima, G.P. Antioxidant activity of gamma-oryzanol: A complex network of interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef]
- Szczesniak, K.A.; Ostaszewski, P.; Ciecierska, A.; Sadkowski, T. Investigation of nutriactive phytochemical-gamma-oryzanol in experimental animal models. J. Anim. Physiol. Anim. Nutr. 2016, 100, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kim, D.; Shimizu, N.; Okada, T.; Hitoe, S.; Shimoda, H. Ninety-day oral toxicity study of rice-derived gamma-oryzanol in sprague-dawley rats. Toxicol. Rep. 2017, 4, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Memo, M.; Uberti, D. Redox homeostasis and natural dietary compounds: Focusing on antioxidants of rice (oryza sativa l.). Nutrients 2018, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Abate, G.; Serafini, M.M.; Guarienti, M.; Catanzaro, M.; Marziano, M.; Memo, M.; Lanni, C.; Uberti, D. Characterization of the antioxidant effects of gamma-oryzanol: Involvement of the nrf2 pathway. Oxid. Med. Cell. Longev. 2018, 2018, 2987249. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Noumi, K.; Sugimoto, I.; Awata, N. Mass fragmentographic determination of ferulic acid in plasma after oral administration of gamma-oryzanol. Chem. Pharm. Bull. 1982, 30, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Sakurai, S.; Sugimoto, I.; Awata, N. Absorption and metabolism of gamma-oryzanol in rats. Chem. Pharm. Bull. 1983, 31, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, C.; Yabiku, K.; Sunagawa, S.; Ueda, R.; Taira, S.; Ohshiro, H.; Ikema, T.; Yamakawa, K.; Higa, M.; Tanaka, H.; et al. Brown rice and its component, gamma-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes 2012, 61, 3084–3093. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Kobayashi, I.; Maruto, S.; Ohshima, K.; Mori, M.; Kamio, N.; Fukuda, H. Effect of gamma-oryzanol on serum tsh concentrations in primary hypothyroidism. Endocrinol. Jpn. 1980, 27, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ieiri, T.; Kase, N.; Hashigami, Y.; Kobori, H.; Nakamura, T.; Shimoda, S. Effects of gamma-oryzanol on the hypothalamo-pituitary axis in the rat. Nihon Naibunpi Gakkai Zasshi 1982, 58, 1350–1356. [Google Scholar] [PubMed]
- Jha, A.B.; Panchal, S.S. Neuroprotection and cognitive enhancement by treatment with γ-oryzanol in sporadic alzheimer’s disease. J. Appl. Biomed. 2017, 15, 265–281. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Tsangaris, G.T.; Maris, A.; Lubec, G. The rat brain hippocampus proteome. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 819, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.; Acsády, L.; Freund, T.F. Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem. Int. 1999, 34, 359–372. [Google Scholar] [CrossRef]
- Albani, S.H.; McHail, D.G.; Dumas, T.C. Developmental studies of the hippocampus and hippocampal-dependent behaviors: Insights from interdisciplinary studies and tips for new investigators. Neurosci. Biobehav. Rev. 2014, 43, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.L.W.; McNair, K.M.; Noble, J.; Brownstein, D.; Hibberd, C.; Mullins, J.J.; Morris, R.G.M.; Cobb, S.; Seckl, J.R.; Morton, N. Enhanced Hippocampal Long-Term Potentiation and Spatial Learning in Aged 11β-Hydroxysteroid Dehydrogenase Type 1 Knock-Out Mice. J. Neurosci. 2007, 27, 10487–10496. [Google Scholar] [CrossRef] [Green Version]
- Gamma-Oryzanol. Available online: https://www.drugs.com/npp/gamma-oryzanol.html (accessed on 25 October 2018).
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.M.; Garrett, L.; Einicke, J.; Sperling, B.; Dirscherl, P.; Zimprich, A.; Fuchs, H.; Gailus-Durner, V.; Hrabe de Angelis, M.; Wurst, W. Assessing cognition in mice. Curr. Protoc. Mouse Biol. 2015, 5, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Wilkening, A.; Rüb, C.; Sylvester, M.; Voos, W. Analysis of heat-induced protein aggregation in human mitochondria. J. Boil. Chem. 2018, 293, 11537–11552. [Google Scholar] [CrossRef]
- Rosenfeld, J.; Capdevielle, J.; Guillemot, J.C.; Ferrara, P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992, 203, 173–179. [Google Scholar] [CrossRef]
- Jenö, P.; Mini, T.; Moes, S.; Hintermann, E.; Horst, M. Internal Sequences from Proteins Digested in Polyacrylamide Gels. Anal. Biochem. 1995, 224, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Käll, L.; Storey, J.D.; MacCoss, M.J.; Noble, W.S. Assigning Significance to Peptides Identified by Tandem Mass Spectrometry Using Decoy Databases. J. Proteome Res. 2008, 7, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Persechini, A.; Cronk, B. The relationship between the free concentrations of Ca2+ and Ca2+-calmodulin in intact cells. J. Biol. Chem. 1999, 274, 6827–6830. [Google Scholar] [CrossRef] [PubMed]
- Shifman, J.M.; Choi, M.H.; Mihalas, S.; Mayo, S.L.; Kennedy, M.B. Ca2+/calmodulin-dependent protein kinase ii (camkii) is activated by calmodulin with two bound calciums. Proc. Natl. Acad. Sci. USA 2006, 103, 13968–13973. [Google Scholar] [CrossRef] [PubMed]
- Ataei, N.; Sabzghabaee, A.M.; Movahedian, A. Calcium/Calmodulin-dependent Protein Kinase II is a Ubiquitous Molecule in Human Long-term Memory Synaptic Plasticity: A Systematic Review. Int. J. Prev. Med. 2015, 6, 88. [Google Scholar]
- Limbäck-Stokin, K.; Korzus, E.; Nagaoka-Yasuda, R.; Mayford, M. Nuclear Calcium/Calmodulin Regulates Memory Consolidation. J. Neurosci. 2004, 24, 10858–10867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlijanian, M.K.; Cooper, D.M. Distinct interactions between Ca2+/calmodulin and neurotransmitter stimulation of adenylate cyclase in striatum and hippocampus. Cell. Mol. Neurobiol. 1988, 8, 459–469. [Google Scholar] [CrossRef]
- Coultrap, S.J.; Bayer, K.U. CaMKII regulation in information processing and storage. Trends Neurosci. 2012, 35, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchesi, W.; Mizuno, K.; Giese, K.P. Novel insights into CaMKII function and regulation during memory formation. Brain Res. Bull. 2011, 85, 2–8. [Google Scholar] [CrossRef]
- Davis, M.M.; Olausson, P.; Greengard, P.; Taylor, J.R.; Nairn, A.C. Regulator of calmodulin signaling (RCS) knockout mice display anxiety-like behavior and motivational deficits. Eur. J. Neurosci. 2012, 35, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Südhof, T.C.; Rizo, J. Synaptic Vesicle Exocytosis. Cold Spring Harb. Perspect. Boil. 2011, 3, a005637. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes snare-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Y.; Liu, J.; Wang, C.; Long, J. Neurodegenerative Disease Related Proteins Have Negative Effects on SNARE-Mediated Membrane Fusion in Pathological Confirmation. Front. Mol. Neurosci. 2017, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, K.J.; Schrod, N.; Davis, T.; Fernández-Busnadiego, R.; Taguchi, Y.V.; Laugks, U.; Lučić, V.; Chandra, S.S. Synucleins have multiple effects on presynaptic architecture. Cell Rep. 2017, 18, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [CrossRef]
- Pelkonen, A.; Yavich, L. Neuromuscular pathology in mice lacking alpha-synuclein. Neurosci. Lett. 2011, 487, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Afanasyeva, M.A.; Van’kin, G.I. Alpha-synuclein knockout mice have cognitive impairments. Behav. Brain Res. 2012, 231, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Victor, K.G.; Heffron, D.S.; Sokolowski, J.D.; Majumder, U.; Leblanc, A.; Mandell, J.W. Proteomic identification of synaptic caspase substrates. Synapse 2018, 72. [Google Scholar] [CrossRef] [PubMed]
- Littleton, J.T.; Barnard, R.J.; Titus, S.A.; Slind, J.; Chapman, E.R.; Ganetzky, B. Snare-complex disassembly by nsf follows synaptic-vesicle fusion. Proc. Natl. Acad. Sci. USA 2001, 98, 12233–12238. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Morgan, A.; Burgoyne, R.D. Stimulation of NSF ATPase Activity by alpha -SNAP Is Required for SNARE Complex Disassembly and Exocytosis. J. Cell Boil. 1997, 139, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Masliah, E.; Mallory, M.; Hansen, L.; DeTeresa, R.; Alford, M.; Terry, R. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci. Lett. 1994, 174, 67–72. [Google Scholar] [CrossRef]
- Xia, Z.; Storm, D.R. The role of calmodulin as a signal integrator for synaptic plasticity. Nat. Rev. Neurosci. 2005, 6, 267–276. [Google Scholar] [CrossRef]
- Gunning, P.; Hardeman, E.; O’Neill, G. Tropomyosin-Based Regulation of the Actin Cytoskeleton in Time and Space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Wu, C.; Chen, L.; Ramirez, A.; Bearer, E.L.; Li, W.-P.; Mobley, W.C.; Chu, S. One at a time, live tracking of ngf axonal transport using quantum dots. Proc. Natl. Acad. Sci. USA 2007, 104, 13666–13671. [Google Scholar] [CrossRef]
- Tai, H.-C.; Besche, H.; Goldberg, A.L.; Schuman, E.M. Characterization of the Brain 26S Proteasome and its Interacting Proteins. Front. Mol. Neurosci. 2010, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Daniels, C.K.; Cao, S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Ther. 2012, 136, 354–374. [Google Scholar] [CrossRef] [PubMed]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. Hspa8/hsc70 chaperone protein: Structure, function, and chemical targeting. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.N.; Furuya, T.K.; Braga, I.L.; Rasmussen, L.T.; Labio, R.W.; Bertolucci, P.H.; Chen, E.S.; Turecki, G.; Mechawar, N.; Payao, S.L.; et al. Analysis of hspa8 and hspa9 mrna expression and promoter methylation in the brain and blood of alzheimer’s disease patients. J. Alzheimer’s Dis. 2014, 38, 165–170. [Google Scholar] [CrossRef]
- McLaughlin, T.; Falkowski, M.; Wang, J.J.; Zhang, S.X. Molecular chaperone ERp29: A potential target for cellular protection in retinal and neurodegenerative diseases. Adv. Exp. Med. Boil. 2018, 1074, 421–427. [Google Scholar]
- Zhang, Y.H.; Belegu, V.; Zou, Y.; Wang, F.; Qian, B.J.; Liu, R.; Dai, P.; Zhao, W.; Gao, F.B.; Wang, L.; et al. Endoplasmic reticulum protein 29 protects axotomized neurons from apoptosis and promotes neuronal regeneration associated with erk signal. Mol. Neurobiol. 2015, 52, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial Fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Boil. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Colangelo, A.M.; Alberghina, L.; Papa, M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci. Lett. 2014, 565, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Tichy, J.; Spechtmeyer, S.; Mittelbronn, M.; Hattingen, E.; Rieger, J.; Senft, C.; Foerch, C. Prospective evaluation of serum glial fibrillary acidic protein (gfap) as a diagnostic marker for glioblastoma. J. Neuro-oncol. 2016, 126, 361–369. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Raza, H. Dual Localization of Glutathione S-Transferase in the Cytosol and Mitochondria: Implications in Oxidative Stress, Toxicity and Disease. FEBS J. 2011, 278, 4243–4251. [Google Scholar] [CrossRef] [Green Version]
- Vannucci, R.C.; Vannucci, S.J. Glucose metabolism in the developing brain. Semin. Perinatol. 2000, 24, 107–115. [Google Scholar] [CrossRef]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular Fatty Acid Metabolism and Cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Klepinin, A.; Ounpuu, L.; Guzun, R.; Chekulayev, V.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Schlattner, U.; Kaambre, T. Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J. Bioenerg. Biomembr. 2016, 48, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Dzeja, P.P.; Chung, S.; Faustino, R.S.; Behfar, A.; Terzic, A. Developmental Enhancement of Adenylate Kinase-AMPK Metabolic Signaling Axis Supports Stem Cell Cardiac Differentiation. PLoS ONE 2011, 6, e19300. [Google Scholar] [CrossRef]
- Sun, M.-K.; Alkon, D.L. Carbonic anhydrase gating of attention: Memory therapy and enhancement. Trends Pharmacol. Sci. 2002, 23, 83–89. [Google Scholar] [CrossRef]
- Sly, W.S.; Hu, P.Y. Human Carbonic Anhydrases and Carbonic Anhydrase Deficiencies. Annu. Rev. Biochem. 1995, 64, 375–401. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Yang, Y.-C.; Tien, C.-P.; Yang, C.-J.; Hsiao, M. Roles of Aldolase Family Genes in Human Cancers and Diseases. Trends Endocrinol. Metab. 2018, 29, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Dalfo, E.; Muntane, G.; Ferrer, I. Glycolitic enzymes are targets of oxidation in aged human frontal cortex and oxidative damage of these proteins is increased in progressive supranuclear palsy. J. Neural Transm. 2008, 115, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Aiba, K.; Kitaura, Y.; Kondo, Y.; Nomura, N.; Nakamura, Y.; Fukushi, D.; Murayama, K.; Shimomura, Y.; Pitt, J.; et al. Clinical, biochemical and metabolic characterisation of a mild form of human short-chain enoyl-coa hydratase deficiency: Significance of increased n-acetyl-s-(2-carboxypropyl)cysteine excretion. J. Med. Genet. 2015, 52, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, B.; Roman, N.; Kobe, B.; Kellie, S.; Forwood, J.K. Functional and structural properties of mammalian acyl-coenzyme A thioesterases. Prog. Lipid Res. 2010, 49, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The Pyruvate Dehydrogenase Complexes: Structure-based Function and Regulation. J. Boil. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, S.; Kikuchi, A.; Takagi, K.; Okuno, J.; Ishikawa, K.; Imada, S.; Horikoshi, T.; Goto, Y.; Hirabayashi, S. A case of pyruvate dehydrogenase e1alpha subunit deficiency with antenatal brain dysgenesis demonstrated by prenatal sonography and magnetic resonance imaging. J. Clin. Ultrasound 2012, 40, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Steller, J.; Gargus, J.J.; Gibbs, L.H.; Hasso, A.N.; Kimonis, V.E. Mild phenotype in a male with pyruvate dehydrogenase complex deficiency associated with novel hemizygous in-frame duplication of the e1alpha subunit gene (pdha1). Neuropediatrics 2014, 45, 56–60. [Google Scholar] [CrossRef]
- Cheng, X.-R.; Zhou, W.-X.; Zhang, Y.-X. The effects of Liuwei Dihuang decoction on the gene expression in the hippocampus of senescence-accelerated mouse. Fitoterapia 2007, 78, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.K.; Reid, C.S.; McMeekin, L.J.; Dougherty, S.E.; Floyd, C.L.; Cowell, R.M. Cerebellar transcriptional alterations with Purkinje cell dysfunction and loss in mice lacking PGC-1α. Front. Cell. Neurosci. 2015, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Bottani, E.; Cerutti, R.; Harbour, M.E.; Ravaglia, S.; Dogan, S.A.; Giordano, C.; Fearnley, I.M.; D’Amati, G.; Viscomi, C.; Fernandez-Vizarra, E.; et al. TTC19 Plays a Husbandry Role on UQCRFS1 Turnover in the Biogenesis of Mitochondrial Respiratory Complex III. Mol. Cell 2017, 67, 96–105. [Google Scholar] [CrossRef]
- Miki, Y.; Tanji, K.; Mori, F.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Alteration of mitochondrial protein PDHA1 in Lewy body disease and PARK14. Biochem. Biophys. Res. Commun. 2017, 489, 439–444. [Google Scholar] [CrossRef]
- Soane, L.; Kahraman, S.; Kristian, T.; Fiskum, G. Mechanisms of Impaired Mitochondrial Energy Metabolism in Acute and Chronic Neurodegenerative Disorders. J. Neurosci. Res. 2007, 85, 3407–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnetti, L.; Reboldi, G.; Gallai, V. Cerebrospinal fluid pyruvate levels in Alzheimer’s disease and vascular dementia. Neurology 2000, 54, 735. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Yoon, H.; Kim, J. Apolipoprotein E metabolism and functions in brain and its role in Alzheimer’s disease. Curr. Opin. Lipidol. 2017, 28, 60–67. [Google Scholar]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72 Pt A, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.L. Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: A review. Atherosclerosis 2016, 255, 145–155. [Google Scholar] [CrossRef]
- Bales, K.R.; Verina, T.; Cummins, D.J.; Du, Y.; Dodel, R.C.; Saura, J.; Fishman, C.E.; DeLong, C.A.; Piccardo, P.; Petegnief, V.; et al. Apolipoprotein e is essential for amyloid deposition in the app(v717f) transgenic mouse model of alzheimer’s disease. Proc. Natl. Acad. Sci. USA 1999, 96, 15233–15238. [Google Scholar] [CrossRef]
- Hyman, B.T.; Holtzman, D.M. Apolipoprotein E levels and Alzheimer risk. Ann. Neurol. 2015, 77, 204–205. [Google Scholar] [CrossRef]
- Bien-Ly, N.; Gillespie, A.K.; Walker, D.; Yoon, S.Y.; Huang, Y. Reducing human apolipoprotein e levels attenuates age-dependent abeta accumulation in mutant human amyloid precursor protein transgenic mice. J. Neurosci. 2012, 32, 4803–4811. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jiang, H.; Park, S.; Eltorai, A.E.; Stewart, F.R.; Yoon, H.; Basak, J.M.; Finn, M.B.; Holtzman, D.M. Haploinsufficiency of human apoe reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J. Neurosci. 2011, 31, 18007–18012. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Brecht, W.J.; Xu, Q.; Tesseur, I.; Kekonius, L.; Wyss-Coray, T.; Fish, J.D.; Masliah, E.; Hopkins, P.C.; Scearce-Levie, K.; et al. Carboxyl-terminal-truncated apolipoprotein e4 causes alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10966. [Google Scholar] [CrossRef]
- Nilsson, L.N.; Arendash, G.W.; Leighty, R.E.; Costa, D.A.; A Low, M.; Garcia, M.F.; Cracciolo, J.R.; Rojiani, A.; Wu, X.; Bales, K.R.; et al. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol. Aging 2004, 25, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.B.; Da, X.; Weiner, M.W.; Wolk, D.A.; Xie, S.X.; Arnold, S.E.; Davatzikos, C.; Shaw, L.M.; Trojanowski, J.Q.; Alzheimer’s Disease Neuroimaging Initiative. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. 2014, 127, 621–632. [Google Scholar] [CrossRef] [Green Version]



| Accession No. | Description | Coverage [%] | MW [kDa] | pI | p Value | ** Fold Change |
|---|---|---|---|---|---|---|
| Synaptic Plasticity and Neuronal Trafficking | ||||||
| Q91ZZ3 | β-Synuclein (Sncb) | 43 | 14.0 | 4.37 | 0.005 | 2.160 |
| O55042 | α-Synuclein (Snca) | 82 | 14.5 | 4.77 | 0.005 | 1.874 |
| P0DP26 | Calmodulin-1 (Calm1) | 52 | 16.8 | 4.22 | 0.003 | 0.799 |
| P21107 | Tropomyosin α-3 chain (Tpm3) | 48 | 33.0 | 4.72 | 0.017 | 1.033 |
| P28663 | Beta-soluble NSF attachment protein (Napb) | 77 | 33.5 | 5.47 | 0.008 | 0.357 |
| P62334 | 26S proteasome regulatory subunit 10B (Psmc6) | 42 | 44.1 | 7.49 | 0.039 | −0.421 |
| Q8R1Q8 | Cytoplasmic dynein 1 light intermediate chain 1 (Dync1li1) | 53 | 56.6 | 6.42 | 0.028 | 1.098 |
| Neuroprotection and antioxidant activity | ||||||
| P10649 | Glutathione S-transferase μ1 (Gstm1) | 59 | 26.0 | 7.94 | 0.009 | 0.677 |
| P57759 | Endoplasmic reticulum resident protein 29 (Erp29) | 36 | 28.8 | 6.15 | 0.039 | 0.694 |
| P03995 | Glial fibrillary acidic protein (Gfap) | 30 | 49.9 | 5.34 | 0.013 | −0.408 |
| P63017 | Heat shock cognate 71 kDa protein (Hspa8) | 52 | 70.8 | 5.52 | 0.007 | 0.319 |
| Mitochondria and energy metabolism | ||||||
| Q9R0Y5 | Adenylate kinase isoenzyme 1 (Ak1) | 47 | 21.5 | 5.81 | 0.000 | 1.308 |
| Q9DBJ1 | Phosphoglycerate mutase 1 (Pgam1) | 80 | 28.8 | 7.18 | 0.000 | 1.463 |
| O70250 | Phosphoglycerate mutase 2 (Pgam2) | 15 | 28.8 | 8.50 | 0.000 | 1.401 |
| P00920 | Carbonic anhydrase 2 (Car2) | 42 | 29.0 | 7.01 | 0.001 | 1.539 |
| Q9CR68 | Cytochrome b-c1 complex subunit Rieske, mitochondrial (Uqcrfs1) | 19 | 29.3 | 8.70 | 0.000 | 2.014 |
| Q8BH95 | Enoyl-CoA hydratase, mitochondrial (Echs1) | 30 | 31.5 | 8.48 | 0.001 | 1.226 |
| P08226 | Apolipoprotein E (ApoE) | 19 | 35.8 | 5.68 | 0.000 | −2.826 |
| P05064 | Fructose-bisphosphate aldolase A (Aldoa) | 61 | 39.3 | 8.09 | 0.001 | 0.413 |
| P35486 | Pyruvate dehydrogenase E1 component subunit α, somatic form, mitochondrial (Pdha1) | 38 | 43.2 | 8.19 | 0.046 | 0.348 |
| Q9QYR9 | Acyl-coenzyme A thioesterase 2, mitochondrial (Acot2) | 35 | 49.6 | 7.36 | 0.048 | 0.548 |
| ** Fold change = log2 [ratio (γ-oryzanol/control)] | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungratanawanich, W.; Cenini, G.; Mastinu, A.; Sylvester, M.; Wilkening, A.; Abate, G.; Bonini, S.A.; Aria, F.; Marziano, M.; Maccarinelli, G.; et al. γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients 2019, 11, 753. https://doi.org/10.3390/nu11040753
Rungratanawanich W, Cenini G, Mastinu A, Sylvester M, Wilkening A, Abate G, Bonini SA, Aria F, Marziano M, Maccarinelli G, et al. γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients. 2019; 11(4):753. https://doi.org/10.3390/nu11040753
Chicago/Turabian StyleRungratanawanich, Wiramon, Giovanna Cenini, Andrea Mastinu, Marc Sylvester, Anne Wilkening, Giulia Abate, Sara Anna Bonini, Francesca Aria, Mariagrazia Marziano, Giuseppina Maccarinelli, and et al. 2019. "γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice" Nutrients 11, no. 4: 753. https://doi.org/10.3390/nu11040753
APA StyleRungratanawanich, W., Cenini, G., Mastinu, A., Sylvester, M., Wilkening, A., Abate, G., Bonini, S. A., Aria, F., Marziano, M., Maccarinelli, G., Memo, M., Voos, W., & Uberti, D. (2019). γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients, 11(4), 753. https://doi.org/10.3390/nu11040753