Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats
Abstract
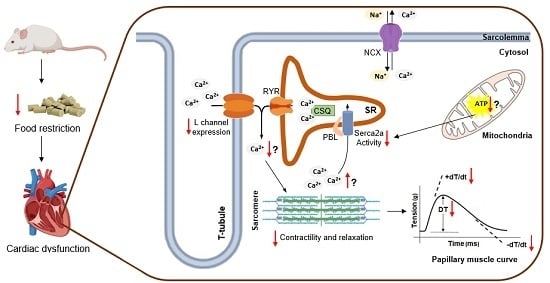
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Protocol
2.2. General Characteristics
2.3. Cardiac Morphological Post Mortem Study
2.4. Myocardial Function
2.5. Expression of Calcium-Handling Protein
2.6. Statistical Analysis
3. Results
3.1. Physical Characteristics
3.2. Macroscopic Cardiac Morphology
3.3. Assessment of Papillary Muscle Function
3.4. Expression of Calcium-Handling Protein
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Alugoju, P.; Narsimulu, D.; Bhanu, J.U.; Satyanarayana, N.; Periyasamy, L. Role of quercetin and caloric restriction on the biomolecular composition of aged rat cerebral cortex: An FTIR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 220, 117128. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trepanowski, J.F.; Canale, E.R.; Marshall, E.K.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Rebrin, I.; Kamzalov, S.; Sohal, R.S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003, 35, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Weindruch, R.; Sohal, R.S. Caloric Intake and Aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Brent, B.; Obonyo, N.; Akech, S.; Shebbe, M.; Mpoya, A.; Mturi, N.; Berkley, J.A.; Tulloh, R.M.R.; Maitland, K. Assessment of Myocardial Function in Kenyan Children with Severe, Acute Malnutrition: The Cardiac Physiology in Malnutrition (CAPMAL) Study. JAMA Netw. Open 2019, 2, e191054. [Google Scholar] [CrossRef] [PubMed]
- Bebars, G.M.; Askalany, H.T. Assessment of left ventricular systolic and diastolic functions in severely malnourished children. Egypt. Pediatr. Assoc. Gaz. 2018. [Google Scholar] [CrossRef] [Green Version]
- Silverman, J.A.; Chimalizeni, Y.; Hawes, S.E.; Wolf, E.R.; Batra, M.; Khofi, H.; Molyneux, E.M. The effects of malnutrition on cardiac function in African children. Arch. Dis. Child. 2016, 101, 166–171. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.L.; Nassar, M.F.; Habib, N.M.; Elmasry, O.A.; Gomaa, S.M. Structural and functional affection of the heart in protein energy malnutrition patients on admission and after nutritional recovery. Eur. J. Clin. Nutr. 2006, 60, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Cicogna, A.C.; Padovani, C.R.; Okoshi, K.; Aragon, F.F.; Okoshi, M.P. Myocardial Function during Chronic Food Restriction in Isolated Hypertrophied Cardiac Muscle. Am. J. Med. Sci. 2000, 320, 244–248. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Okoshi, K.; Pai, V.D.; Pai-Silva, M.D.; Matsubara, L.S.; Cicogna, A.C. Mechanical, biochemical, and morphological changes in the heart from chronic food-restricted rats. Can. J. Physiol. Pharmacol. 2001, 79, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.L.; Okoshi, M.P.; Padovani, C.R.; Aragon, F.F.; Cicogna, A.C. Myocardial dysfunction induced by food restriction is related to calcium cycling and beta-adrenergic system changes. Nutr. Res. 2003, 23, 911–919. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Leopoldo, A.P.L.; Conde, S.J.; Campos, D.S.; Damato, R.; Leopoldo, A.S.; Nascimento, A.F.D.; Júnior, S.D.A.O.; Cicogna, A.C. Upregulation of mRNA myocardium calcium handling in rats submitted to exercise and food restriction. Arq. Bras. Cardiol. 2011, 97, 46–52. [Google Scholar] [CrossRef]
- De Tomasi, L.C.; Bruno, A.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; Nascimento, A.F.; Júnior, S.A.; Pinotti, M.F.; Padovani, C.R.; Leopoldo, A.S.; Cicogna, A.C. Food restriction promotes downregulation of myocardial L-type Ca2+ channels. Can. J. Physiol. Pharmacol. 2009, 87, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.L.; Sugizaki, M.M.; Okoshi, M.P.; Carvalho, R.F.; Pai-Silva, M.D.; Aragon, F.F.; Padovani, C.R.; Okoshi, K.; Cicogna, A.C. Food restriction impairs myocardial inotropic response to calcium and beta-adrenergic stimulation in spontaneously hypertensive rats. Nutr. Res. 2008, 28, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, M.M.; Carvalho, R.F.; Aragon, F.F.; Padovani, C.R.; Okoshi, K.; Okoshi, M.P.; Zanati, S.G.; Pai-Silva, M.D.; Novelli, E.L.B.; Cicogna, A.C. Myocardial Dysfunction Induced by Food Restriction is Related to Morphological Damage in Normotensive Middle-Aged Rats. J. Biomed. Sci. 2005, 12, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Fioretto, J.; Okoshi, M.; Cicogna, A.; Aragon, F.; Matsubara, L.; Matsubara, B. Food restriction induces in vivo ventricular dysfunction in spontaneously hypertensive rats without impairment of in vitro myocardial contractility. Braz. J. Med. Biol. Res. 2004, 37, 607–613. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Leopoldo, A.S.; Okoshi, M.P.; Bruno, A.; Conde, S.J.; Lima-Leopoldo, A.P.; Padovani, C.R.; Carvalho, R.F.; Nascimento, A.F.D.; De Campos, D.H.S.; et al. Severe food restriction induces myocardial dysfunction related to SERCA2 activity. Can. J. Physiol. Pharmacol. 2009, 87, 666–673. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Pai-Silva, M.D.; Carvalho, R.F.; Padovani, C.R.; Bruno, A.; Nascimento, A.F.; Aragon, F.F.; Novelli, E.L.B.; Cicogna, A.C. Exercise training increases myocardial inotropic response in food restricted rats. Int. J. Cardiol. 2006, 112, 191–201. [Google Scholar] [CrossRef]
- Fioretto, J.R.; Querioz, S.S.; Padovani, C.R.; Matsubara, L.S.; Okoshi, K.; Matsubara, B.B. Ventricular remodeling and diastolic myocardial dysfunction in rats submitted to protein-calorie malnutrition. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1327–H1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilderman, T.; McKnight, K.; Dhalla, K.S.; Rupp, H.; Dhalla, N.S. Effects of long-term dietary restriction on cardiovascular function and plasma catecholamines in the rat. Cardiovasc. Drugs Ther. 1996, 10, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Katzeff, H.L.; Powell, S.R.; Ojamaa, K. Alterations in cardiac contractility and gene expression during low-T3 syndrome: Prevention with T3. Am. J. Physiol. Metab. 1997, 273, E951–E956. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Bodell, P.W.; McCue, S.A.; Herrick, R.E.; Baldwin, K.M. Food restriction-induced transformations in cardiac functional and biochemical properties in rats. J. Appl. Physiol. 1993, 74, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.; Savabi, F. Effect of food restriction on the phosphocreatine energy shuttle components in rat heart. J. Mol. Cell. Cardiol. 1992, 24, 821–830. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Okoshi, K.; Matsubara, L.S.; Pai-Silva, M.D.; Gut, A.L.; Padovani, C.R.; Pai, V.D.; Cicogna, A.C. Myocardial remodeling and dysfunction are induced by chronic food restriction in spontaneously hypertensive rats. Nutr. Res. 2006, 26, 567–572. [Google Scholar] [CrossRef]
- Rossi, M.A.; Zucoloto, S. Ultrastructural changes in nutritional cardiomiopathy of protein-calorie malnourished rats. Br. J. Exp. Pathol. 1982, 63, 242–253. [Google Scholar] [PubMed]
- McKnight, K.A.; Rupp, H.; Beamish, R.E.; Dhalla, N.S. Modification of catecholamine-induced changes in heart function by food restriction in rats. Cardiovasc. Drugs Ther. 1996, 10, 239–246. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Shen, H.; Bissonette, D.; Jeejeebhoy, K.N. Effects of hypocaloric feeding and refeeding on myocardial Ca and ATP cycling in the rat. Mol. Cell. Biochem. 1995, 142, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Klebanov, S.; Herlihy, J.T.; Freeman, G.L. Effect of long-term food restriction on cardiac mechanics. Am. J. Physiol. Circ. Physiol. 1997, 273, H:2333–H:2342. [Google Scholar] [CrossRef]
- A Vizotto, V.; Carvalho, R.F.; Sugizaki, M.M.; Lima, A.P.; Aragon, F.F.; Padovani, C.R.; Castro, A.V.B.; Pai-Silva, M.D.; Nogueira, C.R.; Cicogna, A.C. Down-regulation of the cardiac sarcoplasmic reticulum ryanodine channel in severely food-restricted rats. Braz. J. Med. Biol. Res. 2007, 40, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative Analysis of Proteome and Transcriptome Variation in Mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef] [PubMed]
- Foss, E.J.; Radulovic, D.; Shaffer, S.A.; Ruderfer, D.M.; Bedalov, A.; Goodlett, D.R.; Kruglyak, L. Genetic basis of proteome variation in yeast. Nat. Genet. 2007, 39, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.S.; Lima-Leopoldo, A.P.; Sugizaki, M.M.; Nascimento, A.F.D.; De Campos, D.H.S.; Luvizotto, R.D.A.M.; Castardeli, É.; Alves, C.A.B.; Brum, P.C.; Cicogna, A.C.; et al. Involvement of L-type calcium channel and serca2a in myocardial dysfunction induced by obesity. J. Cell. Physiol. 2011, 226, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.H.S.; De Leopoldo, A.S.; Lima-Leopoldo, A.P.; Nascimento, A.F.; Do Oliveira-Junior, S.A.; De Silva, D.C.T.; Sugizaki, M.M.; Padovani, C.R.; Cicogna, A.C. Obesity Preserves Myocardial Function During Blockade of the Glycolytic Pathway. Arq. Bras. Cardiol. 2014, 103, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maxwell, L.; Enwemeka, C.; Fernandes, G. Effects of exercise and food restriction on rat skeletal muscles. Tissue Cell 1992, 24, 491–498. [Google Scholar] [CrossRef]
- Katzeff, H.L.; Ojamaa, K.M.; Klein, I. The effects of long-term aerobic exercise and energy restriction on protein synthesis. Metabolism 1995, 44, 188–192. [Google Scholar] [CrossRef]
- Cicogna, A.C.; Padovani, C.R.; Okoshi, K.; Matsubara, L.S.; Aragon, F.F.; Okoshi, M.P. The influence of temporal food restriction on the performance of isolated cardiac muscle. Nutr. Res. 2001, 21, 639–648. [Google Scholar] [CrossRef]
- Hanft, L.M.; Korte, F.S.; McDonald, K.S. Cardiac function and modulation of sarcomeric function by length. Cardiovasc. Res. 2008, 77, 627–636. [Google Scholar] [CrossRef]
- Sequeira, V.; Nijenkamp, L.L.; Regan, J.A.; Van Der Velden, J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 700–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered myocardial calcium cycling and energetics in heart failure —A rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Janardhan, A.; Efimov, I.R. Remodeling of Calcium Handling in Human Heart Failure. Pharm. Biotechnol. 2012, 740, 1145–1174. [Google Scholar] [Green Version]
- Fan, I.Q.; Chen, B.; Marsh, J.D. Transcriptional Regulation of L-type Calcium Channel Expression in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2000, 32, 1841–1849. [Google Scholar] [CrossRef]
- Golden, K.L.; Ren, J.; Dean, A.; Marsh, J.D. Norepinephrine regulates the in vivo expression of the L-type calcium channel. Mol. Cell. Biochem. 2002, 236, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Wang, D.L.; Chen, W.P.; Hwang, J.J.; Hsieh, C.S.; Hsu, K.L.; Tseng, C.D.; Lai, L.P.; Tseng, Y.Z.; Chiang, F.T.; et al. Angiotensin II Increases Expression of α1C Subunit of L-Type Calcium Channel Through a Reactive Oxygen Species and cAMP Response Element–Binding Protein–Dependent Pathway in HL-1 Myocytes. Circ. Res. 2007, 100, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.; Magyar, J.; Burgess, D.; Andres, D.; Satin, J. Chronic verapamil treatment remodels ICa,L in mouse ventricle. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1906–H1916. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.; Byse, M.; Satin, J. The L-Type Calcium Channel C-terminus Auto-regulates Transcription. Circ. Res. 2009, 104, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Satin, J.; Schroder, E.A.; Crump, S.M. L-type Calcium Channel Auto-Regulation of Transcription. Cell Calcium 2011, 49, 306–313. [Google Scholar] [CrossRef]




| Groups | ||
|---|---|---|
| C (n = 14) | FR (n = 13) | |
| IBW (g) | 313 ± 41.2 | 301 ± 35.2 |
| FBW (g) | 445 ± 39.1 | 228 ± 19.1 * |
| Food intake (g/day) | 21.1 ± 2.2 | 10.6 ± 1.1 |
| Epididymal fat (g) | 9.60 ± 3.42 | 0.90 ± 0.56 * |
| Retroperitoneal fat (g) | 7.00 ± 2.80 | 0.19 ± 0.12 * |
| Visceral fat (g) | 5.24 ± 1.68 | 0.67 ± 0.33 * |
| Total body fat (g) | 21.8 ± 6.90 | 1.75 ± 0.70 * |
| Adiposity index | 4.86 ± 1.45 | 0.76 ± 0.27 * |
| Naso-anal length (cm) | 27.5 ± 0.70 | 24.6 ± 0.80 * |
| Soleus muscle (g) | 0.19 ± 0.03 | 0.10 ± 0.01 * |
| Lung (g) | 2.00 ± 0.41 | 1.19 ± 0.11 * |
| Tibia length (cm) | 4.38 ± 0.07 | 4.15 ± 0.03 * |
| IBW/FBW ratio (g/g) | 0.71 ± 0.10 | 1.33 ± 0.19 * |
| Groups | ||
|---|---|---|
| C (n = 14) | FR (n = 1 3) | |
| LV (g) | 0.84 ± 0.10 | 0.44 ± 0.05 * |
| RV(g) | 0.25 ± 0.04 | 0.12 ± 0.01 * |
| AT (g) | 0.10 ± 0.02 | 0.05 ± 0.01 * |
| Total heart(g) | 1.20 ± 0.15 | 0.62 ± 0.07 * |
| LV/FBW (mg/g) | 1.90 ± 0.22 | 1.93 ± 0.13 |
| RV/FBW (mg/g) | 0.56 ± 0.08 | 0.53 ± 0.03 |
| AT/FBW (mg/g) | 0.24 ± 0.04 | 0.24 ± 0.02 |
| Heart/FBW (mg/g) | 2.70 ± 0.32 | 2.70 ± 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deus, A.F.d.; Silva, V.L.d.; de Souza, S.L.B.; Mota, G.A.F.; Sant’Ana, P.G.; Vileigas, D.F.; Lima-Leopoldo, A.P.; Leopoldo, A.S.; Campos, D.H.S.d.; de Tomasi, L.C.; et al. Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients 2019, 11, 1985. https://doi.org/10.3390/nu11091985
Deus AFd, Silva VLd, de Souza SLB, Mota GAF, Sant’Ana PG, Vileigas DF, Lima-Leopoldo AP, Leopoldo AS, Campos DHSd, de Tomasi LC, et al. Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients. 2019; 11(9):1985. https://doi.org/10.3390/nu11091985
Chicago/Turabian StyleDeus, Adriana Fernandes de, Vítor Loureiro da Silva, Sérgio Luiz Borges de Souza, Gustavo Augusto Ferreira Mota, Paula Grippa Sant’Ana, Danielle Fernandes Vileigas, Ana Paula Lima-Leopoldo, André Soares Leopoldo, Dijon Henrique Salomé de Campos, Loreta Casquel de Tomasi, and et al. 2019. "Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats" Nutrients 11, no. 9: 1985. https://doi.org/10.3390/nu11091985
APA StyleDeus, A. F. d., Silva, V. L. d., de Souza, S. L. B., Mota, G. A. F., Sant’Ana, P. G., Vileigas, D. F., Lima-Leopoldo, A. P., Leopoldo, A. S., Campos, D. H. S. d., de Tomasi, L. C., Padovani, C. R., Kolwicz, S. C., Jr., & Cicogna, A. C. (2019). Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients, 11(9), 1985. https://doi.org/10.3390/nu11091985