Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being
Abstract
:1. Introduction
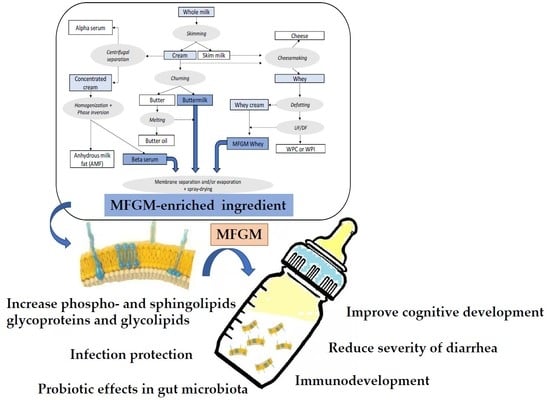
2. Sources, Production, and Treatments of Dairy-Based Ingredients Containing Milk Fat Globule Membrane (MFGM)
3. Biological Effects of MFGM
3.1. Proteins of the MFGM
3.2. Lipids of the MFGM
3.3. Preclinical Evidence on MFGM Components and Ingredients
- Anti-pathogenic effects
- Intestinal epithelial, mucosal immune system, and gut microbiota
- Neurodevelopment
4. Comments on Clinical Studies
5. MFGM-Enriched Dairy Ingredients: Critical Unit Operations and Safety Concerns
6. Regulatory and Safety Aspects of Infant Formula (IF) Added Ingredients
7. Future Developments
- Better characterization of minor MFGM proteins and their content along with their variability, as influenced by the processing conditions of the ingredients.
- Demonstrate and ensure that MFGM systems deliver bioactivities to the infants in a reproducible manner (process control vs. variability of raw material).
- Develop sustainable processes as alternatives to solvent extraction to increase the phospholipid or minor lipid content from MFGM-enriched ingredients.
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality. A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Jimenez-Flores, R. Symposium review: The relevance of bovine milk phospholipids in human nutrition-Evidence of the effect on infant gut and brain development. J. Dairy Sci. 2019, 102, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Ahern, G.J.; Hennessy, A.A.; Ryan, C.A.; Ross, R.P.; Stanton, C. Advances in Infant Formula Science. Annu. Rev. Food Sci. Technol. 2019, 10, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Unraveling the Complexity of Milk Fat Globules to Tailor Bioinspired Emulsions Provid/ing Health Benefits: The Key Role Played by the Biological Membrane. Eur. J. Lipid Sci. Technol. 2019, 121, 1800201. [Google Scholar]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lonnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, L.; van Dijk, G.; van der Beek, E.M. Milk lipid composition and structure; The relevance for infant brain development. OCL Oilseeds Fats Crops Lipids 2020, 27, 5. [Google Scholar] [CrossRef] [Green Version]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Loennerdal, B. Efficacy of an MFGM-enriched Complementary Food in Diarrhea, Anemia, and Micronutrient Status in Infants. J. Parenter. Enteral Nutr. 2011, 53, 561–568. [Google Scholar]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Comment on “Safety and Tolerance Evaluation of Milk Fat Globule Membrane-Enriched Infant Formulas: A Randomized Controlled Multicenter Non-Inferiority Trial in Healthy Term Infants”. Clinical Medicine Insights. Pediatrics 2015, 9, 63–64. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Stenlund, H.; Lönnerdal, B.; Hernell, O. Feeding Infants Formula with Probiotics or Milk Fat Globule Membrane: A Double-Blind, Randomized Controlled Trial. Front. Pediatr. 2019, 7, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Mather, I.; Keenan, T.W. Origin and secretion of milk lipids. J. Mamm. Gland Biol. Neopl. 1998, 3, 259–273. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, M.; Lönnerdal, B.; Hernell, O. Supplementation of Infant Formula with Bovine Milk Fat Globule Membranes. Adv. Nutr. 2017, 15, 351–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosqvist, F.; Smedman, A.; Lindmark-Mansson, H.; Paulsson, M.; Petrus, P.; Straniero, S.; Rudling, M.; Dahlman, I.; Riserus, U. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. Am. J. Clin. Nutr. 2015, 102, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, T.S.; Demmer, E.; Rivera, N.; Gertz, E.R.; German, J.B.; Smilowitz, J.T.; Zivkovic, A.M.; Van Loan, M.D. The role of a dairy fraction rich in milk fat globule membrane in the suppression of postprandial inflammatory markers and bone turnover in obese and overweight adults: An exploratory study. Nutr. Metab. 2017, 14, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higurashi, S.; Haruta-Ono, Y.; Urazono, H.; Kobayashi, T.; Kadooka, Y. Improvement of skin condition by oral supplementation with sphingomyelin-containing milk phospholipids in a double-blind, placebo-controlled, randomized trial. J. Dairy Sci. 2015, 98, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ten Bruggencate, S.J.; Frederiksen, P.D.; Pedersen, S.M.; Floris-Vollenbroek, E.G.; Lucas-van de Bos, E.; van Hoffen, E.; Wejse, P.L. Dietary Milk-Fat-Globule Membrane Affects Resistance to Diarrheagenic Escherichia coli in Healthy Adults in a Randomized, Placebo-Controlled, Double-Blind Study. J. Nutr. 2016, 146, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.C.; Tomé-Carneiro, J.; Gómez-Coronado, D.; Burgos-Ramos, E.; García-Serrano, A.; Martín-Hernández, R.; Baliyan, S.; Fontecha, J.; Venero, C.; Dávalos, A.; et al. Modulation of miRNA expression in aged rat hippocampus by buttermilk and krill oil. Sci. Rep. 2018, 8, 3993. [Google Scholar] [CrossRef] [Green Version]
- Tomé-Carneiro, J.; Carmen Crespo, M.; Burgos-Ramos, E.; Tomas-Zapico, C.; García-Serrano, A.; Castro-Gómez, P.; Venero, C.; Pereda-Pérez, I.; Baliyan, S.; Valencia, A.; et al. Buttermilk and krill oil phospholipids improve hippocampal insulin resistance and synaptic signaling in aged rats. Mol. Neurobiol. 2018, 55, 7285–7296. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Jarén-Galán, M.; Garrido-Fernández, J.; Calvo, M.V.; Visioli, F.; Fontecha, J. Activities, bioavailability, and metabolism of lipids from structural membranes and oils: Promising research on mild cognitive impairment. Pharmacol. Res. 2018, 134, 299–304. [Google Scholar] [CrossRef]
- García-Serrano, A.; Tomé-Carneiro, J.; Crespo, M.C.; Visitación Calvo, M.V.; Pereda-Pérez, I.; Baliyan, S.; Burgos-Ramos, E.; Montero, O.; Dávalos, A.; Venero, C.; et al. Concentrates of buttermilk and krill oil improve spatial memory in aged rats. Prostaglandins Leukot. Essent. Fatty Acids 2020, 155, 102077. [Google Scholar] [CrossRef]
- Abrams, S.A.; Daniels, S.R. Protecting Vulnerable Infants by Ensuring Safe Infant Formula Use. J. Pediatr. 2019, 211, 201–206. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, H.; Guan, W.; Liu, J.; Brennan, C.; Kulasiri, D.; Mohan, M.S. Vesicle properties and health benefits of milk phospholipids: A review. J. Food Bioact. 2019, 5, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Gallier, S. Nature’s complex emulsion: The fat globules of milk. Food Hydrocoll. 2017, 68, 81–89. [Google Scholar] [CrossRef]
- Gallier, S.; Vocking, K.; Post, J.A.; Van de Heijning, B.; Acton, D.; Van der Beek, E.M.; Van Baalen, T. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Coll. Surf. B-Biointerfaces 2015, 136, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Organization of lipids in milks, infant milk formulas and various dairy products: Role of technological processes and potential impacts. Dairy Sci. Technol. 2015, 95, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fatty Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Jiménez-Flores, R.; Brisson, G. The milk fat globule membrane as an ingredien: Why, how, when? Dairy Sci. Technol. 2008, 88, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Singh, H. The milk fat globule membrane—A biophysical system for food applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 154–163. [Google Scholar] [CrossRef]
- de Boer, R. From Milk by-Products to Milk Ingredients—Upgrading the Cycle; John Wiley & Sons Ltd.: West Sussex, UK, 2014; p. 269. [Google Scholar]
- Price, N.; Fei, T.; Clark, S.; Wang, T. Extraction of phospholipids from a dairy by-product (whey protein phospholipid concentrate) using ethanol. J. Dairy Sci. 2018, 101, 8778–8787. [Google Scholar] [CrossRef]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, Sweden, 2015. [Google Scholar]
- Huang, Z.; Zheng, H.; Brennan, C.S.; Mohan, M.S.; Stipkovits, L.; Li, L.; Kulasiri, D. Production of Milk Phospholipid-Enriched Dairy. Foods 2020, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Rombaut, R.; Dewettinck, K. Thermocalcic aggregation of milk fat globule membrane fragments from acid buttermilk cheese whey. J. Dairy Sci. 2007, 90, 2665–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumy, J.J.; Gestin, L.; Fauquant, J.; Boyaval, E.; Maubois, J.L. Technologies de purification des phospholipides du lactoserum. Process 1990, 1047, 29–33. [Google Scholar]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Castro-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2020, 86, 3. [Google Scholar] [CrossRef]
- Holzmueller, W.; Kulozik, U. Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. Int. Dairy J. 2016, 63, 88–91. [Google Scholar] [CrossRef]
- Rombaut, R.; Dewettinck, K. Properties, analysis and purification of milk polar lipids. Int. Dairy J. 2006, 16, 1362–1373. [Google Scholar] [CrossRef]
- Britten, M.; Lamothe, S.; Robitaille, G. Effect of cream treatment on phospholipids and protein recovery in butter-making process. Int. J. Food Sci. Technol. 2008, 43, 651–657. [Google Scholar] [CrossRef]
- Konrad, G.; Kleinschmidt, T.; and Lorenz, C. Ultrafiltration of whey buttermilk to obtain a phospholipid concentrate. Int. Dairy J. 2013, 30, 39–44. [Google Scholar] [CrossRef]
- Morin, P.; Pouliot, Y.; Jiménez-Flores, R. A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. J. Food Eng. 2006, 77, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Haddadian, Z.; Eyres, G.T.; Carne, A.; Everett, D.W.; Bremer, P. Impact of different milk fat globule membrane preparations on protein composition, xanthine oxidase activity, and redox potential. Int. Dairy J. 2017, 64, 14–21. [Google Scholar] [CrossRef]
- Haddadian, Z.; Eyres, G.T.; Bremer, P.; and Everett, D.W. Polar lipid composition of the milk fat globule membrane in buttermilk made using various cream churning conditions or isolated from commercial samples. Int. Dairy J. 2018, 81, 138–142. [Google Scholar] [CrossRef]
- Le, T.T.; Debyser, G.; Gilbert, W.; Struijs, K.; Van Camp, J.; Van de Wiele, T.; Devreese, B.; Dewettinck, K. Distribution and isolation of milk fat globule membrane proteins during dairy processing as revealed by proteomic analysis. Int. Dairy J. 2013, 32, 110–120. [Google Scholar] [CrossRef]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-Depth Lipidomic Analysis of Molecular Species of Triacylglycerides, Diacylglycerides, Glycerophospholipids, and Sphingolipids of Buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, R.; Camp, J.V.; Dewettinck, K. Analysis of Phospho- and Sphingolipids in Dairy Products by a New HPLC Method. J. Dairy Sci. 2005, 88, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Bourlieu, C.; Cheillan, D.; Blota, M.; Daira, P.; Trauchessec, M.; Ruet, S.; Gassi, J.-Y.; Beaucher, E.; Robert, B.; Leconte, N.; et al. Polar lipid composition of bioactive dairy co-products buttermilk and butterserum: Emphasis on sphingolipid and ceramide isoforms. Food Chem. 2018, 240, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, C.; Blot, M.; Briard-Bion, V.; Cirie, C.; Graulet, B. Butter serums and buttermilks as sources of bioactive lipids from the milk fat globule membrane: Differences in their lipid composition and potentialities of cow diet to increase n-3 PUFA. Food Res. Int. 2017, 100, 864–872. [Google Scholar] [CrossRef]
- Lopez, C.; Cauty, C.; Rousseau, F.; Blot, M.; Margolis, A.; Famelart, M.H. Lipid droplets coated with milk fat globule membrane fragments: Microstructure and functional properties as a function of pH. Food Res. Int. 2017, 91, 26–37. [Google Scholar] [CrossRef]
- Gassi, J.Y.; Blot, M.; Beaucher, E.; Robert, B.; Leconte, N.; Camier, B.; Rousseau, F.; Bourlieu, C.; Jardin, J.; Briard-Bion, V.; et al. Preparation and characterisation of a milk polar lipids enriched ingredient from fresh industrial liquid butter serum: Combination of physico-chemical modifications and technological treatments. Int. Dairy J. 2016, 52, 26–34. [Google Scholar] [CrossRef]
- Lambert, S.; Leconte, N.; Blot, M.; Rousseau, F.; Robert, B.; Camier, B.; Gassi, J.Y.; Cauty, C.; Lopez, C.; Gésan-Guiziou, G. The lipid content and microstructure of industrial whole buttermilk and butter serum affect the efficiency of skimming. Food Res. Int. 2016, 83, 121–130. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. The temperature-dependent physical state of polar lipids and their miscibility impact the topography and mechanical properties of bilayer models of the milk fat globule membrane. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2181–2190. [Google Scholar] [CrossRef]
- Smoczynski, M.; Staniewski, B.; Kielczewska, K. Composition and Structure of the Bovine Milk Fat Globule Membrane-Some Nutritional and Technological Implications. Food Rev. Int. 2012, 28, 188–202. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Duc Ninh, N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Bourlieu, C.; Michalski, M.-C. Structure-function relationship of the milk fat globule. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 118–127. [Google Scholar] [CrossRef]
- Douëllou, T.; Montel, M.C.; Sergentet, D.T. Anti-adhesive properties of bovine oligosaccharides and bovine milk fat globule membrane-associated glycoconjugates against bacterial food enteropathogens. J. Dairy Sci. 2017, 100, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Le Huerou-Luron, I.; Lemaire, M.; Blat, S. Health benefits of dairy lipids and MFGM in infant formula. OCL Oilseeds Fats Crops Lipids 2018, 25, 7. [Google Scholar] [CrossRef] [Green Version]
- Guerin, J.; Burgain, J.; Gomand, F.; Scher, J.; Gaiani, C. Milk fat globule membrane glycoproteins: Valuable ingredients for lactic acid bacteria encapsulation? Crit. Rev. Food Sci. Nutr. 2019, 59, 639–651. [Google Scholar] [CrossRef]
- Le Huerou-Luron, I.; Lemaire, M.; Blat, S. Health benefits of dairy lipids and MFGM in infant formula. Cah. Nutr. Diet. 2019, 54, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Holzmüller, W.; Kulozik, U. Quantification of MFGM proteins in buttermilk and butter serum by means of a stain free SDS-PAGE method. J. Food Compos. Anal. 2016, 49, 102–109. [Google Scholar] [CrossRef]
- Holzmueller, W.; Mueller, M.; Himbert, D.; Kulozik, U. Impact of cream washing on fat globules and milk fat globule membrane proteins. Int. Dairy J. 2016, 59, 52–61. [Google Scholar] [CrossRef]
- Parker, P.; Sando, L.; Pearson, R.; Kongsuwan, K.; Tellam, R.L.; Smith, S. Bovine Muc1 inhibits binding enteric bacteria to Caco-2 cells. Glycoconj J. 2010, 27, 89–97. [Google Scholar] [CrossRef]
- Kvistgaard, A.S.; Pallesen, L.T.; Arias, C.F.; Lopez, S.; Petersen, T.E.; Heegaard, C.W.; Rasmussen, J.T. Inhibitory effects of human and bovine milk constituents on rotavirus infections. J. Dairy Sci. 2004, 87, 4088–4096. [Google Scholar] [CrossRef] [Green Version]
- Yolken, R.H.; Peterson, J.A.; Vonderfecht, S.L.; Fouts, E.T.; Midthun, K.; Newburg, D.S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Invest. 1992, 90, 1984–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.T. Bioactivity of milk fat globule membrane proteins. Aust. J. Dairy Technol. 2009, 64, 63–67. [Google Scholar]
- Martin, H.M.; Hancock, J.T.; Salisbury, V.; Harrison, R. Role of Xanthine Oxidoreductase as an Antimicrobial Agent. Infect. Immun. 2004, 72, 4933–4939. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R. Milk xanthine oxidase: Properties and physiological roles. Int. Dairy J. 2006, 16, 546–554. [Google Scholar] [CrossRef]
- Berer, K.; Schubart, A.; Williams, K.; Linington, C. Pathological consequences of molecular mimicry between myelin oligodendrocyte glycoprotein (MOG) and butyrophilin (BTN) in experimental autoimmune encephalomyelitis (EAE). Immunology 2005, 116, 42. [Google Scholar]
- Steffer, A.; Schubart, A.; Storch, M.; Amini, A.; Mather, I.; Lassmann, H.; Linington, C. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. Baltim. 2000, 165, 2859–2865. [Google Scholar] [CrossRef] [Green Version]
- Vojdani, A.; Campbell, A.W.; Anyanwu, E.; Kashanian, A.; Bock, K.; Vojdani, E. Antibodies to neuron-specific antigens in children with autism: Possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumoniae and Streptococcus group A. J. Neuroimmunol. 2002, 129, 168–177. [Google Scholar] [CrossRef]
- Rhodes, D.A.; Reith, W.; Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; de Lourdes Guerrero, M.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protectoin against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Riccio, P. The proteins of the milk fat globule membrane in the balance. Trends Food Sci. Technol. 2004, 15, 458–461. [Google Scholar] [CrossRef]
- Spitsberg, V.L.; Matitashvili, E.; Gorewit, R.C. Association and coexpression of fatty-acid-binding protein and glycoprotein cd36 in the bovine mammary-gland. Eur. J. Biochem. 1995, 230, 872–878. [Google Scholar] [CrossRef]
- Gorewit, R.C.; Spitsberg, V.L. Anti-Cancer Properties of Proteins in the Milk fat Globule Membranes in Whey. International Dairy Federation. In Whey International Conference; International Dairy Federation: Brussels, Belgium, 1998; pp. 315–325. [Google Scholar]
- Mather, I.H. A review and proposed nomenclature for major proteins of the milk-fat globule membrane. J. Dairy Sci. 2000, 83, 203–247. [Google Scholar] [CrossRef]
- Hancke, K.; Grubeck, D.; Hauser, N.; Kreienberg, R.; Weiss, J.M. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Res. Treat. 2010, 119, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Mochizuki, K.; Honma, K.; Miyauchi, R.; Kasezawa, N.; Tohyama, K.; Goda, T. Serum Fatty Acid Binding Protein 4 Concentrations Are Positively and Independently Associated with Blood Pressure and Abdominal Fat among Parameters in Health Check-Ups in Ordinary Middle-Aged Japanese Males. J. Nutr. Sci. Vitaminol. 2015, 61, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillaud, E.; Bastuji-Garin, S.; Plonquet, A.; Foucat, E.; Fournier, B.; Boutin, E.; Le Thuaut, A.; Levy, Y.; Hue, S. Combined Plasma Elevation of CRP, Intestinal-Type Fatty Acid-Binding Protein (I-FABP), and sCD14 Identify Older Patients at High Risk for Health Care-Associated Infections. J. Gerontol. Ser. A 2018, 73, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Egbuche, O.; Biggs, M.L.; Ix, J.; Kizer, J.; Lyles, M.; Siscovick, D.; Djousse, L.; Mukamal, K. Fatty acid binding protein-4 and risk of cardiovascular disease: The cardiovascular health study. J. Am. Coll. Cardiol. 2019, 73, 1833. [Google Scholar] [CrossRef]
- Daniels, M.J.; Wang, Y.; Lee, M.; Venkitaraman, A.R. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 2004, 306, 876–879. [Google Scholar] [CrossRef]
- Vissac, C.; Lemery, D.; Le Corre, L.; Fustier, P.; Dechelotte, P.; Maurizis, J.-C.; Bignon, Y.-J.; Bernard-Gallon, D.J. Presence of BRCA1 and BRCA2 proteins in human milk fat globules after delivery. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2002, 1586, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Parron, A.J.; Ripolles, D.; Perez, M.D.; Calvo, M.; Rasmussen, J.T.; Sanchez, L. Antirotaviral Activity of Bovine and Ovine Dairy Byproducts. J. Agric. Food Chem. 2017, 65, 4280–4288. [Google Scholar] [CrossRef]
- Zhao, L.L.; Du, M.; Gao, J.; Zhan, B.Y.; Mao, X.Y. Label-free quantitative proteomic analysis of milk fat globule membrane proteins of yak and cow and identification of proteins associated with glucose and lipid metabolism. Food Chem. 2019, 275, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cong, M.; Peng, X.; Wu, J.; Wu, R.; Liu, B.; Ye, W.; Yue, X. Quantitative proteomic analysis of milk fat globule membrane (MFGM) proteins in human and bovine colostrum and mature milk samples through iTRAQ labeling. Food Funct. 2016, 7, 2438–2450. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gómez, M.P.; Rodriguez-Alcalá, L.M.; Calvo, M.V.; Romero, J.; Mendiola, J.A.; Ibañez, E.; Fontecha, J. Total milk fat extraction and quantification of polar and neutral lipids of cow, goat, and ewe milk by using a pressurized liquid system and chromatographic techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilla, A.; Diego-Quintaes, K.; Barberá, R.; Alegría, A. Phospholipids in human milk and infant formulas: Benefits and needs for correct infant nutrition. Crit. Rev. Food Sci. Nutr. 2016, 56, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem. 2012, 135, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Cichello, F.; Ragonese, C.; Donato, P.; Cacciola, F.; Dugo, P.; Mondello, L. Profiling and quantifying polar lipids in milk by hydrophilic interaction liquid chromatography coupled with evaporative light-scattering and mass spectrometry detection. Anal. Bioanal. Chem. 2013, 405, 4617–4626. [Google Scholar] [CrossRef]
- Zou, X.-Q.; Guo, Z.; Huang, J.-H.; Jin, Q.-Z.; Cheong, L.-Z.; Wang, X.-G.; Xu, X.-B. Human milk fat globules from different stages of lactation: A lipid composition analysis and microstructure characterization. J. Agric. Food Chem. 2012, 60, 7158–7167. [Google Scholar] [CrossRef]
- Corthésy, B. Secretory immunoglobulin A: Well beyond immune exclusion at mucosal surfaces. Immunopharmacol. Immunotoxicol. 2009, 31, 174–179. [Google Scholar] [CrossRef]
- Claumarchirant, L.; Cilla, A.; Matencio, E.; Sanchez-Siles, L.M.; Castro-Gomez, P.; Fontecha, J.; Alegría, A.; Lagarda, M.J. Addition of Milk Fat Globule Membrane as an Ingredient of Infant Formulas for Resembling the Polar Lipids of Human Milk. Int. Dairy J. 2016, 61, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Giuffrida, F.; Cruz-Hernandez, C.; Bertschy, E.; Fontannaz, P.; Masserey Elmelegy, I.; Tavazzi, I. Temporal changes of human breast milk lipids of Chinese mothers. Nutrients 2016, 8, 715. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; MacGibbon, A.K.H.; Jan Mohamed, H.J.B.; Loy, S.L.; Rowan, A.; McJarrow, P.; Fong, B.Y. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography–mass spectrometry–multiple reaction monitoring method. Int. Dairy J. 2017, 71, 50–59. [Google Scholar] [CrossRef]
- MacFarland, B.; Bettler, J.; Moloney, C.; O’Regan, J.; Giuffrida, F.; Thakkar, S.; Lee, L.Y. Sphingomyelin Content in Breast Milk and Infant Formula: A Nutrient That May Affect Neurodevelopment. Adv. Hum. Nutr. 2017, 8, 1–17. [Google Scholar]
- Tavazzi, I.; Fontannaz, P.; Lee, L.Y.; Giuffrida, F. Quantification of glycerophospholipids and sphingomyelin in human milk and infant formula by high performance liquid chromatography coupled with mass spectrometer detector. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1072, 235–243. [Google Scholar] [CrossRef]
- Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B: Lipid quality in infant nutrition: Current knowledge and future opportunities. J. Pediatr Gastroenterol. Nutr. 2015, 61, 8–17.
- Castro-Gómez, P.; Rodríguez-Alcalá, L.M.; Monteiro, K.M.; Ruiz, A.L.T.G.; Carvalho, J.E.; Fontecha, J. Antiproliferative activity of buttermilk lipid fractions isolated using food grade and non-food grade solvents on human cancer cell lines. Food Chem. 2016, 212, 695–702. [Google Scholar] [CrossRef]
- Fauquant, C.; Briard-Bion, V.; Leconte, N.; Guichardarit, M.; Michalski, M.-C. Membrane phospholipids and sterols in microfiltered milk fat globules. Eur. J. Lipid Sci. Technol. 2007, 109, 1167–1173. [Google Scholar] [CrossRef]
- Lopez, C.; Menard, O. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B 2011, 83, 29–41. [Google Scholar] [CrossRef]
- Arranz, E. and M. Corredig Milk phospholipid vesicles, their colloidal properties, and potential as delivery vehicles for bioactive molecules. J. Dairy Sci. 2017, 100, 4213–4222. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Ma, B.; Song, S.; Lai, O.-M.; Cheong, L.-Z. Fingerprinting of Phospholipid Molecular Species from Human Milk and Infant Formula Using HILIC-ESI-IT-TOF-MS and Discriminatory Analysis by Principal Component Analysis. J. Agric. Food Chem. 2018, 66, 7131–7138. [Google Scholar] [CrossRef]
- Brink, Lonnerdal 2020- JNB. (in review process).
- Vickers, M.H.; Guan, J.; Gustavsson, M.; Krageloh, C.U.; Breier, B.H.; Davison, M.; Hodgkinson, S.C. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutr. Res. 2009, 29, 426–435. [Google Scholar] [CrossRef]
- Guan, J.; MacGibbon, A.; Fong, B.; Zhang, R.; Liu, K.; Rowan, A.; McJarrow, P. Long-Term Supplementation with Beta Serum Concentrate (BSC), a Complex of Milk Lipids, during Post-Natal Brain Development Improves Memory in Rats. Nutrients 2015, 7, 4526–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, L.R.; Lonnerdal, B. The role of milk fat globule membranes in behavior and cognitive function using a suckling rat pup supplementation model. J. Nutr. Biochem. 2018, 58, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Brink, L.R.; Gueniot, J.P.; Lönnerdal, B. Effects of milk fat globule membrane and its various components on neurologic development in a postnatal growth restriction rat model. J. Nutr. Biochem. 2019, 69, 163–171. [Google Scholar] [CrossRef]
- Moukarzel, S.; Dyer, R.A.; Garcia, C.; Wiedeman, A.M.; Boyce, G.; Weinberg, J.; Keller, B.O.; Elango, R.; Innis, S.M. Milk Fat Globule Membrane Supplementation in Formula-fed Rat Pups Improves Reflex Development and May Alter Brain Lipid Composition. Sci. Rep. 2018, 8, 15277. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; McVey Neufeld, K.A.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C.; et al. The enduring effects of early-life stress on the microbiota-gut-brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur. J. Neurosci. 2020, 51, 1042–1058. [Google Scholar] [CrossRef]
- Mudd, A.T.; Alexander, L.S.; Berding, K.; Waworuntu, R.V.; Berg, B.M.; Donovan, S.M.; Dilger, R.N. Dietary Prebiotics, Milk Fat Globule Membrane, and Lactoferrin Affects Structural Neurodevelopment in the Young Piglet. Front. Pediatr. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Fil, J.E.; Fleming, S.A.; Chichlowski, M.; Gross, G.; Berg, B.M.; Dilger, R.N. Evaluation of Dietary Bovine Milk Fat Globule Membrane Supplementation on Growth, Serum Cholesterol and Lipoproteins and Neurodevelopment in the Young Pig. Front. Pediatr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sprong, R.C.; Hulstein, M.F.; Lambers, T.T.; van der Meer, R. Sweet buttermilk intake reduces colonisation and translocation of Listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. Br. J. Nutr. 2012, 108, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Snow, D.R.; Jimenez-Flores, R.; Ward, R.E.; Cambell, J.; Young, M.J.; Nemere, I.; Hintze, K.J. Dietary Milk Fat Globule Membrane Reduces the Incidence of Aberrant Crypt Foci in Fischer-344 Rats. J. Agric. Food Chem. 2010, 58, 2157–2163. [Google Scholar] [CrossRef]
- Snow, D.R.; Ward, R.E.; Olsen, A.; Jimenez-Flores, R.; Hintze, K.J. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. J. Dairy Sci. 2011, 94, 2201–2212. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wu, Z.; Liu, C.; Han, D.; Feng, C.; Wang, S.; Wang, J. Milk Fat Globule Membrane Supplementation Promotes Neonatal Growth and Alleviates Inflammation in Low-Birth-Weight Mice Treated with Lipopolysaccharide. BioMed. Res. Int. 2019, 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, J.; Niu, Y.; Chen, H.; Tang, Q.; Zhong, Y.; Lambers, T.T.; Cai, W. Milk Fat Globule Membrane Inhibits NLRP3 Inflammasome Activation and Enhances Intestinal Barrier Function in a Rat Model of Short Bowel. J. Parenter. Enteral. Nutr. 2019, 43, 677–685. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, J.; Zhou, J.; Cai, W.; Qian, L. Milk Fat Globule Membrane Ameliorates Necrotizing Enterocolitis in Neonatal Rats and Suppresses Lipopolysaccharide-Induced Inflammatory Response in IEC-6 Enterocytes. J. Parenter Enteral Nutr. 2019. [Google Scholar] [CrossRef]
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, M.B.; Miller, M.J.; Dilger, R.N.; et al. Prebiotics and Bioactive Milk Fractions Affect Gut Development, Microbiota, and Neurotransmitter Expression in Piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697. [Google Scholar] [CrossRef]
- Thompson, R.S.; Roller, R.; Mika, A.; Greenwood, B.N.; Knight, R.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Dietary Prebiotics and Bioactive Milk Fractions Improve NREM Sleep, Enhance REM Sleep Rebound and Attenuate the Stress-Induced Decrease in Diurnal Temperature and Gut Microbial Alpha Diversity. Front. Behav. Neurosci. 2016, 10, 240. [Google Scholar] [CrossRef]
- Bhinder, G.J.; Allaire, M.; Garcia, C.; Lau, J.T.; Chan, J.M.; Ryz, N.R.; Bosman, E.S.; Graef, F.A.; Crowley, S.M.; Celiberto, L.S.; et al. Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development. Sci. Rep. 2017, 7, 45274. [Google Scholar] [CrossRef]
- Nejrup, R.G.; Licht, T.R.; Hellgren, L.I. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Sci. Rep. 2017, 7, 3975. [Google Scholar] [CrossRef] [Green Version]
- Huërou-Luron, I.; Bouzerzour, K.; Ferret-Bernard, S.; Ménard, O.; Normand, L.; Perrier, C.; Dupont, D. A mixture of milk and vegetable lipids in infant formula changes gut digestion, mucosal immunity and microbiota composition in neonatal piglets. Eur. J. Nutr. 2018, 57, 463–476. [Google Scholar] [CrossRef]
- Otnaess, A.B.; Laegreid, A.; Ertresvag, K. Inhibition of enterotoxin from Escherichia coli and Vibrio cholerae by gangliosides from human milk. Infect. Immun. 1983, 40, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Schroten, H.; Hanisch, F.G.; Plogmann, R.; Hacker, J.; Uhlenbruck, G.; Nobis-Bosch, R.; Wahn, V. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: A novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect. Immun. 1992, 60, 2893–2899. [Google Scholar] [CrossRef] [Green Version]
- Idota, T.; Kawakami, H.; Murakami, Y.; Sugawara, M. Inhibition of cholera toxin by human milk fractions and sialyllactose. Biosci. Biotechnol. Biochem. 1995, 59, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.R.; Millar, T.M.; Clinch, J.G.; Kanczler, J.M.; Bodamyali, T.; Blake, D.R. Antibacterial properties of xanthine oxidase in human milk. Lancet 2000, 356, 829–830. [Google Scholar] [CrossRef]
- Tellez, A.; Corredig, M.; Guri, A.; Zanabria, R.; Griffiths, M.W.; Delcenserie, V. Bovine milk fat globule membrane affects virulence expression in Escherichia coli O157:H7. J. Dairy Sci. 2012, 95, 6313–6319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novakovic, P.; Huang, Y.Y.; Lockerbie, B.; Shahriar, F.; Kelly, J.; Gordon, J.R.; Simko, E. Identification of Escherichia coli F4ac-binding proteins in porcine milk fat globule membrane. Can. J. Vet. Res. 2015, 79, 120–128. [Google Scholar]
- Douëllou, T.; Galia, W.; Kerangart, S.; Marchal, T.; Milhau, N.; Bastien, R.; Bouvier, M.; Buff, S.; Montel, M.-C.; Sergentet-Thevenot, D. Milk Fat Globules Hamper Adhesion of Enterohemorrhagic Escherichia coli to Enterocytes: In Vitro and in Vivo Evidence. Front. Microbiol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Guri, A.; Griffiths, M.; Khursigara, C.M.; Corredig, M. The effect of milk fat globules on adherence and internalization of Salmonella Enteritidis to HT-29 cells. J. Dairy Sci. 2012, 95, 6937–6945. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Z.; Chen, C.; Kling, D.E.; Newburg, D.S. Human milk mucin 1 and mucin 4 inhibit Salmonella enterica serovar Typhimurium invasion of human intestinal epithelial cells in vitro. J. Nutr. 2012, 142, 1504–1509. [Google Scholar] [CrossRef] [Green Version]
- Fuller, K.L.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Jimenez-Flores, R.; Donovan, S.M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 2013, 96, 3488–3497. [Google Scholar] [CrossRef] [Green Version]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Bu, H.F.; Zuo, X.L.; Wang, X.; Ensslin, M.A.; Koti, V.; Hsueh, W.; Raymond, A.S.; Shur, B.D.; Tan, X.D. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J. Clin. Invest. 2007, 117, 3673–3683. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.J.; Gao, J.; Yang, H.M.; Yuan, X.L.; Chen, T.X.; He, Z.J. The role of the lactadherin in promoting intestinal DCs development in vivo and vitro. Clin. Dev. Immunol. 2010, 357541. [Google Scholar] [CrossRef]
- Milard, M.; Penhoat, A.; Durand, A.; Buisson, C.; Loizon, E.; Meugnier, E.; Bertrand, K.; Joffre, F.; Cheillan, D.; Garnier, L.; et al. Acute effects of milk polar lipids on intestinal tight junction expression: Towards an impact of sphingomyelin through the regulation of IL-8 secretion. J. Nutr. Biochem. 2019, 65, 128–138. [Google Scholar] [CrossRef]
- Anderson, R.C.; MacGibbon, A.K.H.; Haggarty, N.; Armstrong, K.M.; Roy, N.C. Bovine dairy complex lipids improve in vitro measures of small intestinal epithelial barrier integrity. PLoS ONE 2018, 13, e0190839. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.; Barratt, M.J.; Gordon, J.I. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Rueda, R.; Sabatel, J.L.; Maldonado, J.; Molina-Font, J.A.; Gil, A. Addition of gangliosides to an adapted milk formula modifies levels of fecal Escherichia coli in preterm newborn infants. J. Pediatr. 1998, 133, 90–94. [Google Scholar] [CrossRef]
- Popov, N.; Toffano, G.; Riechert, U.; Matthies, H. Effects of intraventricularly applied gangliosides and N-acetylneuraminic acid on acquisition and retention performance of a brightness discrimination task in rats. Pharmacol. Biochem. Behav. 1989, 34, 209–212. [Google Scholar] [CrossRef]
- Mei, Z.T.; Zheng, J.Z. Effects of exogenous gangliosides on learning and memory in rats. Jpn. J. Physiol. 1993, 43, S295–S299. [Google Scholar]
- Fujii, S.; Igarashi, K.; Sasaki, H.; Furuse, H.; Ito, K.; Kaneko, K.; Ando, S. Effects of the mono- and tetrasialogangliosides GM1 and GQ1b on ATP-induced long-term potentiation in hippocampal CA1 neurons. Glycobiology 2002, 12, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 2003, 53, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Wu, Y.P.; Sandhoff, R.; Werth, N.; Mizukami, H.; Ellis, J.M.; Proia, R.L. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 2725–2730. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H. The fetal origins of memory: The role of dietary choline in optimal brain development. J. Pediatr. 2006, 149, S131–S136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susuki, K.; Baba, H.; Tohyama, K.; Kanai, K.; Kuwabara, S.; Hirata, K.; Yuki, N. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia 2007, 55, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.R.; Kim, H.G.; Kim, K.L. Ganglioside GQ1b improves spatial learning and memory of rats as measured by the Y-maze and the Morris water maze tests. Neurosci. Lett. 2008, 439, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.; Montalto, M.; Ponder, D. Lower Incidence of Necrotizing Enterocolitis in Infants Fed a Preterm Formula with Egg Phospholipids. Pediatr. Res. 1998, 44, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zavaleta, N.; Chen, S.-Y.; Lonnerdal, B.; Slupsky, C. Effect of bovine milk fat globule membranes as a complementary food on the serum metabolome and immune markers of 6-11-month-old Peruvian infants. NPJ Sci. Food 2018, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013, 35, 45–52. [Google Scholar] [CrossRef]
- Poppitt, S.D.; McGregor, R.A.; Wiessing, K.R.; Goyal, V.K.; Chitkara, A.J.; Gupta, S.; Palmano, K.; Kuhn-Sherlock, B.; McConnell, M.A. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern Indian infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Timby, N.; Domellof, E.; Hernell, O.; Lonnerdal, B.; Domellof, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Timby, N.; Lonnerdal, B.; Hernell, O.; Domellof, M. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr Res. 2014, 76, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timby, N.; Hernell, O.; Vaarala, O.; Melin, M.; Lönnerdal, B.; Domellöf, M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. A randomized controlled trial. J. Pediatr Gastroenterol. Nutr. 2015, 60, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Domellof, M.; Holgerson, P.L.; West, C.E.; Lonnerdal, B.; Hernell, O.; Johansson, I. Oral Microbiota in Infants Fed a Formula Supplemented with Bovine Milk Fat Globule Membranes—A Randomized Controlled Trial. PLoS ONE 2017, 12, e0169831. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Parenti, M.; Grip, T.; Domellof, M.; Lonnerdal, B.; Hernell, O.; Timby, N.; Slupsky, C.M. Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: A randomized controlled trial. Sci. Rep. 2019, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Billeaud, C.; Puccio, G.; Saliba, E.; Guillois, B.; Vaysse, C.; Pecquet, S.; Steenhout, P. Safety and tolerance evaluation of milk fat globule membrane-enriched infant formulas: A randomized controlled multicenter non-inferiority trial in healthy term infants. Clinical medicine insights. Pediatrics 2014, 8, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Lukoyanova, O.; Borovik, T.; Bushueva, T.; Stepanova, T.; Zvonkova, N.; Melnichuk, O.; Kopyltsova, E. The lipid metabolism in infants fed formula supplemented with bovine milk fat and bovine milk fat globule membranes. In: Abstracts of the 50th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; Prague. J. Pediatr. Gastroenterol. Nutr. 2017, 66 (Suppl. 1), 801. [Google Scholar]
- Gallier, S.; Xia, Y.; Rowan, A.; Wang, B. Milk fat globule membrane as a source of gangliosides and phospholipids in infancy to support brain development and healthy growth. In: Abstracts of the 51th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; Geneva. J. Pediatr. Gastroenterol. Nutr. 2018, 66 (Suppl. 2), 942. [Google Scholar]
- Demmelmair, H.; Uhl, O.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Prosser, C.; Koletzko, B. Plasma sphingomyelins of infants differ according to source of milk fat In Abstracts of the 51th Annual Meeting of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition; Geneva. J. Pediatr. Gastroenterol. Nutr. 2018, 66 (Suppl. 2), 941. [Google Scholar]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef]
- Nieto-Ruiz, A.; García-Santos, J.A.; Bermúdez, M.G.; Herrmann, F.; Diéguez, E.; Sepúlveda-Valbuena, N.; García, S.; Miranda, M.T.; De-Castellar, R.; Rodréguez-Palmero, M.; et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients 2019, 11, 2456. [Google Scholar] [CrossRef] [Green Version]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.-J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Parenti, M.; Grip, T.; Lonnerdal, B.; Timby, N.; Domellof, M.; Hernell, O.; Slupsky, C.M. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: A randomized controlled trial. Sci. Rep. 2019, 9, 11589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grip, T.; Dyrlund, T.S.; Ahonen, L.; Domellof, M.; Hernell, O.; Hyotylainen, T.; Knip, M.; Lonnerdal, B.; Oresic, M.; Timby, N. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatr. Res. 2018, 84, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Institute of Medicine (US). Infant Formula: Evaluating the Safety of New Ingredients; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products‚ Nutrition and Allergies). Guidance on the preparation and presentation of an application for authorization of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, 4594. [Google Scholar] [CrossRef] [Green Version]
- Guidelines on EFSA Consultations. Eur. Food Saf. Auth. 2018, 15, 1390E. [CrossRef] [Green Version]
- Schmitt, J.; Spuls, P.I.; Thomas, K.S.; Simpson, E.; Furue, M.; Deckert, S.; Dohil, M.; Apfelbacher, C.; Singh, J.A.; Chalmers, J.; et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. HOME initiative collaborators. J. Allergy Clin. Immunol. 2014, 134, 800–807. [Google Scholar] [CrossRef]
- Mirotti, L.; Florsheim, E.; Rundqvist, L.; Larsson, G.; Spinozzi, F.; Leite-de-Moraes, M.; Russo, M.; Alcocer, M. Lipids are required for the development of Brazil nut allergy: The role of mouse and human iNKT cells. Allergy 2012, 68, 74–83. [Google Scholar] [CrossRef]


| MFGM-Enriched Ingredients | |||
|---|---|---|---|
| (g/100 g Product) | Buttermilk | Beta Serum | Whey |
| Source/process | Butter | Anhydrous milk fat | Cheese whey |
| Protein (N × 6.38) | ≥30 | >52 | 73 |
| Lactose | ±50 | ≤10 | ≤3 |
| Ash | ≤9 | ≤6 | ≤3 |
| Total lipids | 5–13 | 3–27 | 12–26 |
| Total phospholipids (PL) (g/100g fat) | 1.6–22 | ≥14 | 5–16 |
| Phospholipids (% of total PL) | |||
| - Phosphatidyl ethanolamine (PE) | 35–43 | 22–29 | 19–41 |
| - Phosphatidyl choline (PC) | 19–32 | 27–47 | 19–25 |
| - Phosphatidyl serine (PS) | 8–18 | 1.2–23 | 8–12 |
| - Phosphatidyl inositol (PI) | 4–9 | 1–8 | 3.6–7 |
| - Sphingomyelin (SM) | 11–19 | 14–27 | 16–24 |
| - Others | - | <4 | <7 |
| Moisture | ≤4% | ≤5% | ≤6% |
| pH | 6.0–6.5 | 6.2–6.8 | 6.4 |
| Protein Common Name | Molecular Weight |
|---|---|
| BRCA1 and BRCA2 | 210 |
| Mucin I (MUC1) | 160–200 |
| Xanthine oxidase (XO) | 146–155 |
| PAS III | 94–100 |
| CD36 | 76–78 |
| Butyrophilin (BTN) | 66–67 |
| Adipophilin (ADPH) | 52 |
| PAS 6/7 (lactadherin) | 47–59 |
| Proteose peptone 3 | 18–34 |
| FABP | 13–15 |
| Component | Health Aspects | References |
|---|---|---|
| Studies in vitro and in vivo | ||
| Mucin I (Muc1) | Antiadhesive effect | [62] |
| Protective effect against rotavirus infection | [63,64] | |
| Effects on digestion | [65] | |
| Xanthine dehydrogenase/ | Antimicrobial agent | [66] |
| oxidase (XDH/XO) | Source of reactive oxygen species (ROS)/anti-inflammatory properties | [67] |
| Butyrophilin (BTN) | Suppression of multiple sclerosis | [68] |
| Development of experimental autoimmune encephalomyelitis | [69] | |
| Influence on pathogenesis of autistic behavior | [70] | |
| Regulation of immunity | [71] | |
| Periodic acid Schiff 6/7 (PAS 6/7) (lactadherin) | Protection from viral infections in the gut | [63,72] |
| Epithelialization, cell polarization, cell movement and rearrangement, neurite outgrowth, synaptic activity in the central nervous system | [73] | |
| Fatty acid binding | Breast cancer cells lines inhibition | [74,75,76] |
| Protein (FABP) | Association with breast cancer; indicator | [77,78,79,80] |
| Cluster of differentiation (CD36) | Anticancer properties by interacting with FABP | [75] |
| Anti-inflammatory properties | [54] | |
| Breast cancer susceptibility proteins (BRCA1 and BRCA2) | Breast cancer DNA repair process inhibition | [81,82] |
| MFGM proteins (complex) | Prevention of diarrhea and improvement of anemia | [7] |
| Retroviral infection prevention | [83] | |
| Lipid digestion and cholesterol absorption reduction | [55] | |
| Proteomic studies of MFGM in their complexity | Association and correlation studies with health or nutrition | [84] |
| Comparative study of human and cow proteins | [85] | |
| Ingredient Description | Model | Design | Primary Finding | Ref |
|---|---|---|---|---|
| MFGM and brain development | ||||
| Complex milk lipid (CML) | Young rats | Oral supplementation via gavage from PD10–80 | Improved Novel Object and Morris Water Maze performance | [104] |
| CML-Beta serum concentrate (BSC) | Young rats | Provided orally from PD10–60 as a gelatin | Reduced latency in Morris Water Maze test; increased expression of striatal dopamine terminals and hippocampal glutamate receptors | [105] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rat pups | Oral supplementation MFGM ingredient at 500 mg/kg BW until PND21 | Improved neurodevelopment (increased gene expression of BDNF and glutamate-receptor) and improved behavior test performance | [106] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rat pups | Oral supplementation with WPC, MFGM ingredient, phospholipid concentrate (PL-20), and sialic acid | Increased hippocampal expression corresponding to improved behavior performance in adulthood | [107] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rat pups | MFGM via cannulas inserted into the stomach | Brain metabolite differences along with improved reflexes (ear and eyelid twitch, negative geotaxis, and cliff avoidance) | [108] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rat pups | MFGM in combination with prebiotics and subjected to stress via maternal separation | Ameliorated stress-induced visceral hypersensitivity and improved gut-brain axis response to stress | [109] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) in a formula | Piglets | Mixture of lactoferrin, MFGM ingredient, and polydextrose galacto-oligosaccharides (PDX/GOS) for 30 days | T microvascular changes in the brain related to grey matter concentration and diffusivity within the internal capsule | [110] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Piglets | Milk replacer with 0, 2.5, or 5 g/L of MFGM ad libitum access | Higher serum cholesterol and HDL in MFGM-2.5 g/L; No differences in brain cholesterol or changes in brain macro/micro-structure | [111] |
| MFGM and immune/intestinal development | ||||
| Sweet buttermilk powder MFGM | Rats | Buttermilk powder enriched in food pre- and during infection | Reduced colonization and translocation of the pathogenic bacteria | [112] |
| Cream-derived MFGM | Rats | MFGM incorporated into diet for a period of 12 weeks; colon carcinogenesis rat model | Provided resistance to gut insult through significantly less aberrant crypt foci | [113] |
| Cream-derived MFGM | Mice | MFGM into diet for 5 weeks and injected with LPS | More resilient to intestinal inflammation, lower levels of inflammatory cytokines, and less intestinal permeability | [114] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Neonatal mouse | MFGM ingredient daily during suckling period and treated with a lipopolysaccharides challenge on postnatal day 21 | Less inflammation (lower inflammatory cytokines, GI hist score, higher expression of GJ proteins) | [115] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rats | MFGM ingredient from day 2–14 post operation 75–85% small bowel resection | Lower expression of inflammatory cytokines and NLRP3 inflammasome | [116] |
| Bovine MFGM with unspecified starting material | Rats | MFGM supplemented with two concentrations in a model of Necrotizing enterocolitis (NEC) | Higher concentration group (12 g/L) exhibited reduced intestinal injury (lower NEC score, lower inflammatory cytokines, and improved survival rates) | [117] |
| Whey-derived MFGM (MFGM-10 Lacproda ®) in a formula | Neonatal piglet | Mixture of lactoferrin, MFGM ingredient, and polydextrose galacto- oligosaccharides for 30 days | Improved GI development (increased enzyme activity and morphology) and lower pathogenic bacteria in the colon | [118] |
| MFGM and the establishment of the microbiota | ||||
| Whey-derived MFGM (MFGM-10 Lacprodan®) in a formula | Neonatal rat | Mixture of MFGM, lactoferrin, and prebiotics in a stress model | Improvements in sleep and protection against growth of Lactobacillus rhamnosus | [119] |
| Whey-derived MFGM (MFGM-10 Lacprodan ®) | Rat pups | MFGM in combination with prebiotics in a maternal separation stress model | No differences | [109] |
| Whey-derived MFGM (MFGM-10 Lacprodan®) | Rat pups | Formula with MFGM via cannulas inserted into the stomach’ challenged with C. difficile toxin | MFGM protective against C. difficile toxin damage | [120] |
| Whey-derived phospholipid concentrate (PL-20 Lacprodan®) | Mice | PL-20 was compared with soy lecithin for use as an emulsifier on human microbiota-colonized germ-free mice | PL showed significantly higher relative abundance of multiple bacteria and concentrations of short chain fatty acids | [121] |
| Whey-derived MFGM (MFGM-10 Lacprodan®) in a formula | Neonatal piglet | Mixture of MFGM, lactoferrin, and polydextrose galacto-oligosaccharides for 30 days | Increased abundance of Clostridium IV, Parabacteroides, Lutispora, and Sutterella, which was associated with increased total body weight | [118] |
| MFGM fragments from undescribed source | Neonatal piglet | Formula with MFGM fragments compared with milk fat and with vegetable oils | MFGM had increased Proteobacteria and Bacteroidetes and decreased Firmicutes; changes in mucosal immunity were also observed | [122] |
| MFGM Type * | Methods | Results | Safety | Study |
|---|---|---|---|---|
| GNGL | Preterm infants (32–36 weeks) fed IF with added gangliosides (GMF, n = 20) (1.43 mg/100 kcal) compared to (–) gangliosides (MF, n = 20) for 4 weeks | Reduced levels of Escherichia coli in the feces of GMF compared to MF at postnatal day 7; higher fecal counts of Bifidobacteria at postnatal day 30 in GMF | None: No adverse events or growth differences were reported. | [139] |
| PL from egg | IF provided to preterm infants +/– added PLs (+n = 34) (–n = 85) in the hospital; development of NEC was observed | Infants fed PL formula developed less NEC stage II and III with similar rates of bronchopulmonary dysplasia, septicemia, and retinopathy of prematurity. | None: No difference in weight gain or formula consumption was observed. | [149] |
| WPC | MFGM-10 enriched formula fed daily for 6 months to infants aged 6–11 months (5.9 g/day) (n = 253); skim milk control (n = 246) | Lower prevalence of diarrhea (3.8% MFGM vs. 4.4% skim); 46% reduction of episodes of bloody diarrhea | None: No difference in growth or serum markers (ferritin, zinc, or folate) were observed. | [7,150] |
| GNGL CML | IF with GNGL enriched CML (n = 29) fed to healthy infants (2–8 weeks old) until six months old; compared to non-supplemented formula (n = 30) and BF reference group (n = 32) | Test group had increased behavioral test scores on the Griffiths Mental Development Scale at 6 months. | None: No differences in growth or tolerance between the two formula groups. | [151] |
| SM | IF with SM (n = 12) or control (n = 13) fed for 8 weeks to low birth weight infants (<1500 g); evaluations up to 18 months | Increased SM in total PLs and improved scores on Behavior Rating Scale of the BSID-II, the Fagan test scores, the latency of visual evoked potentials, and sustained attention test scores at 18 months | None: No adverse events or growth differences were reported. | [152] |
| GNGL CML | Supplement 2 g CML + 3 g whole-milk power or control 5 g whole-milk powder for 12 weeks to infants age 8–24 months | Lower duration of rotavirus diarrhea and prevalence of major illness in the CML group was observed. | None: There were no difference in adverse events or growth between groups. | [153] |
| WPC | IF supplemented with MFGM-10 at 6g/L (n = 80), standard formula (n = 80), and BF reference group (BF) (n = 72) fed to term infants <2 months old until 6 months of age | MFGM-fed group showed significantly higher mean cognitive domain scores vs. the control group at 12 months of age; overall phenotypes observed to be more similar to BF group | None: No difference in eczema or any skin rash reported. MFGM formula supported growth and was well tolerated. Lower incidence of otitis media and antipyretic drug use reported. Serum antibody levels were more similar to BF group. | [154,155,156,157,158] |
| WPC | IF supplemented with MFGM-10 (n = 72), MFGM-L “lipid rich” (n = 70), or SF control (n = 57) fed to infants ≤14 days old at enrollment and provided until 4 months of age. | No significant differences were found for plasma PLs, cardiolipin, cholesterol ester, IGF-1, or leptin. No differences were observed in polio or HiB antibodies, with the exception of lower mean polio virus type 1 IgG level in the MFGM-10 in one group. No differences were found between groups in fecal immune markers, including alpha-1-antitrypsin, secretory IgA, and calprotectin. | No difference in adverse events or growth and tolerance. However, post-hoc analysis reported rates of eczema were higher in MFGM-10® group (13.9% vs. 3.5% in SF). The short duration and relatively small sample size, as well as the unequal allocation of subjects among groups, which may have introduced some degree of bias between groups. | [159] |
| Cream MFGM | IF supplemented with MFGM concentrate compared to control formula and BF reference from <2 months of age and 2 months of consumption. | Concentration of serum LDL and cholesterol in MFGM-fed infants was comparable to the BF reference group. | None: No adverse events were reported. | [160] |
| CML MFMG | IF with MFGM (n = 226) fed to full term infants <14 days old up to 12 months and compared to BF reference group (n = 206). | Growth and dropout rate had similar scores between FF infants and BF reference at birth and 6 months. Behavioral tests, serum GNGL, and gut microbiota were measured. | None: No differences in weight between groups were reported. | [161] |
| MFGM within goat milk fat | IF containing either goat milk fat/plant oil mixture (GIF) or control formula with only plant oil fat provided to healthy infants up to 4 months of age. | Significantly different SM and fatty acids patterns were observed between groups. However, the profiles did not directly represent the dietary fatty acid pattern. | None: No differences between groups in weight gain or adverse events was observed. | [162] |
| WPC | IF, SF with MFGM-10 or with probiotic (L. paracasei ssp. paracasei strain F19) along with a BF reference group (n = 200 per group) fed for 4 months and followed to 12 months. | MFGM formula group did not have significantly more diarrhea, fever, days with fever, clinic visits, or URI episodes than the other formula groups or the BF infants. | None: Both experimental formulas were well tolerated and supported normal growth. Adverse event rates were highest in the control formula group with significantly more fever episodes and days with fever than the BF reference group. | [9] |
| WPC | IF provided 2 weeks through 12 months with either SF (n = 208) or MFGM-10 at 5 g/L with Lactoferrin (0.6 g/L (n = 198). Children were followed to 18 months of age. | MFGM+Lf formula group had higher cognitive, language, and motor Bayley-III scores at 12 months, better sustained attention at 12 months, and higher scores on some elements of language performance at 18 months, and were not inferior to the control group in any neurodevelopmental measure. | None: No significant differences between groups in growth, intolerance, fussiness, or stool characteristics were observed. Adverse event analysis demonstrated no differences between groups in antibiotic use and significantly lower incidences of GI and respiratory adverse events (including diarrhea, URI, and cough) for the MFGM + Lf formula group vs. the control group. | [163] |
| WPC + other bioactive ingredients | IF added MFGM-10, GNGL, LC-PUFA’s, sialic acid, and synbiotics (n = 85) compared to a control formula (n = 85) and BF reference group (n = 50). Term infants fed from <2 months of age until 18 months. | There were no differences in growth or neurodevelopment between formula groups; however, visual function was found to be more similar to the BF reference group. | None: No difference in weight/length gain between formula groups was observed. | [164] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. https://doi.org/10.3390/nu12061607
Fontecha J, Brink L, Wu S, Pouliot Y, Visioli F, Jiménez-Flores R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients. 2020; 12(6):1607. https://doi.org/10.3390/nu12061607
Chicago/Turabian StyleFontecha, Javier, Lauren Brink, Steven Wu, Yves Pouliot, Francesco Visioli, and Rafael Jiménez-Flores. 2020. "Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being" Nutrients 12, no. 6: 1607. https://doi.org/10.3390/nu12061607
APA StyleFontecha, J., Brink, L., Wu, S., Pouliot, Y., Visioli, F., & Jiménez-Flores, R. (2020). Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients, 12(6), 1607. https://doi.org/10.3390/nu12061607