Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Extraction
2.3. Gas Chromatograph-Mass Spectrometer/Mass Spectrometer (GC-MS/MS) Analysis
2.4. Cell Line
2.5. MTT Assay
2.6. ROS Assay
2.7. Cell Cycle Assay by Flow Cytometer
2.8. Apoptosis Assay by Flow Cytometer
2.9. Protein Expression by Western Blotting
2.10. Molecular Docking
2.10.1. Ligand Preparation
2.10.2. Protein Preparation
2.10.3. Molecular Docking
2.11. Statistical Analysis
3. Results
3.1. Effects of the Extract and Polycyclic Aromatic Hydrocarbons (PAHs) on Cell Viability
3.2. Effects of the MC Extract on PAHs-Induced Cytotoxicity and Oxidative Stress
3.3. Effects of MC Extract on PAHs-Induced Apoptosis and Cell Cycle Arrest
3.4. Effects of MC Extract on Apoptotic- and Cell Cycle-Associated Protein Expression
3.5. Metabolite Profiling of Ethanolic Extract of MC by GC-MS/MS
3.6. The Ability of MC-Derived Phytochemical Constituents in a Role as the Inhibitors of CYP Isomers Using an In Silico Approach
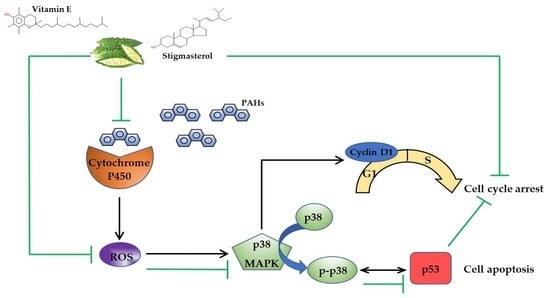
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Tongo, I.; Ogbeide, O.; Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017, 4, 55–61. [Google Scholar] [CrossRef]
- Peterson, B.S.; Rauh, V.A.; Bansal, R.; Hao, X.; Toth, Z.; Nati, G.; Walsh, K.; Miller, R.L.; Arias, F.; Semanek, D.; et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015, 72, 531–540. [Google Scholar] [CrossRef]
- Blazkova, B.; Pastorkova, A.; Solansky, I.; Veleminsky, M., Jr.; Veleminsky, M.; Urbancova, K.; Vondraskova, V.; Hajslova, J.; Pulkrabova, J.; Sram, R.J. Effect of Polycyclic Aromatic Hydrocarbons exposure on cognitive development in 5 years old children. Brain Sci. 2020, 10, 619. [Google Scholar] [CrossRef]
- Rao, P.S.S.; Kumar, S. Polycyclic aromatic hydrocarbons and cytochrome P450 in HIV pathogenesis. Front. Microbiol. 2015, 6, 550. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Al-Attas, O.S.; Al-Daghri, N.M.; Mohammed, A.A.; De Rosas, E.; Ibrahim, S.; Vinodson, B.; Ansari, M.G.; El-Din, K.I.A. Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol. Cell. Biochem. 2014, 391, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.N.; L’Allemain, G.; Brunet, A.; Müller, R.; Pouysségur, J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 1996, 271, 20608–20616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanovas, O.; Miró, F.A.; Estanyol, J.M.; Itarte, E.; Agell, N.; Bachs, O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 2000, 275, 35091–35097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Kim, N.J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [Green Version]
- Efird, J.T.; Choi, Y.M.; Davies, S.W.; Mehra, S.; Anderson, E.J.; Katunga, L.A. Potential for improved glycemic control with dietary Momordica charantia in patients with insulin resistance and prediabetes. Int. J. Environ. Res. Public Health 2014, 11, 2328–2345. [Google Scholar] [CrossRef] [Green Version]
- Rahmatullah, M.; Azam, N.K.; Khatun, Z.; Seraj, S.; Islam, F.; Rahman, M.A.; Jahan, S.; Aziz, M.S. Medicinal plants used for treatment of diabetes by the Marakh sect of the Garo tribe living in Mymensingh district, Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.Y.; Yi, Y.; Zhang, L.F.; Zhang, R.F.; Zhang, Y.; Wei, Z.C.; Tang, X.J.; Zhang, M.W. Immunomodulatory activity and partial characterisation of polysaccharides from Momordica charantia. Molecules 2014, 19, 13432–13447. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.I.C.; Ling, A.P.K.; Koh, R.Y.; Chye, S.M.; Voon, K.G.L. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Aljohi, A.; Matou-Nasri, S.; Ahmed, N. Antiglycation and antioxidant properties of Momordica charantia. PLoS ONE 2016, 11, e0159985. [Google Scholar] [CrossRef]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent advances in momordica charantia: Functional components and biological activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattarachotanant, N.; Tencomnao, T. Citrus hystrix extracts protect human neuronal cells against high glucose-induced senescence. Pharmaceuticals 2020, 13, 283. [Google Scholar] [CrossRef]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef] [Green Version]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Savas, U.; Stout, C.D.; Johnson, E.F. Structural characterization of the complex between alpha-naphthoflavone and human cytochrome P450 1B1. J. Biol. Chem. 2011, 286, 5736–5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Sugiura, H.; Tanaka, H.; Takigami, S.; Yamagata, K. P38 MAP kinase inhibitors as potential therapeutic drugs for neural diseases. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 45–59. [Google Scholar] [CrossRef]
- Albertini, R.; Abuja, P.M. Prooxidant and antioxidant properties of Trolox C, analogue of vitamin E, in oxidation of low-density lipoprotein. Free Radic. Res. 2009, 30, 181–188. [Google Scholar] [CrossRef]
- Sagach, V.F.; Scrosati, M.; Fielding, J.; Rossoni, G.; Galli, C.; Visioli, F. The water-soluble vitamin E analogue Trolox protects against ischaemia/reperfusion damage in vitro and ex vivo. A comparison with vitamin E. Pharmacol. Res. 2002, 45, 435–439. [Google Scholar] [CrossRef]
- Takahashi, E.; Fujita, K.I.; Kamataki, T.; Arimoto-Kobayashi, S.; Okamoto, K.; Negishi, T. Inhibition of human cytochrome P450 1B1, 1A1 and 1A2 by antigenotoxic compounds, purpurin and alizarin. Mutat. Res. 2002, 508, 147–156. [Google Scholar] [CrossRef]
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Gürdal, B.; Kültür, S. An ethnobotanical study of medicinal plants in Marmaris (Mugla, Turkey). J. Ethnopharmacol. 2013, 146, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Bolognesi, A. Plants producing ribosome-inactivating proteins in traditional medicine. Molecules 2016, 21, 1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Thornton, T.M.; Rincon, M. Non-classical P38 Map kinase function: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–52. [Google Scholar] [CrossRef]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, J.W. A Method of Geometrical Representation of the Thermodynamic Properties of Substances by Means of Surfaces, 2nd ed.; Transactions of Connecticut Academy of Arts and Sciences: London, UK, 1873; pp. 382–404. [Google Scholar]










| Compound Name | Nature of Compound | RT (Min) | Area (%) | MF | MW |
|---|---|---|---|---|---|
| Ketone | |||||
| 1,2-cyclopentanedione | Lactone | 3.649 | 0.56 | C5H6O2 | 98 |
| d-galactonic acid, γ-lactone | Galactonolactone | 14.936 | 0.16 | C6H10O6 | 178 |
| Alcohol | |||||
| Glycerin | Polyol compound | 3.974 | 1.09 | C3H8O3 | 92 |
| Phytol | Diterpene alcohol | 17.649 | 1.03 | C20H40O | 296 |
| Aromatic aldehyde | |||||
| Benzeneacetaldehyde | 4.976 | 0.16 | C8H8O | 120 | |
| Ester | |||||
| Ethyl hydrogen malonate | Malonic acid ester | 5.518 | 2.77 | C5H8O4 | 132 |
| Hexadecanoic acid, methyl ester | Fatty acid methyl ester | 15.632 | 0.28 | C17H34O2 | 270 |
| Hexadecanoic acid, ethyl ester | Fatty acid ethyl ester | 16.303 | 1.4 | C18H36O2 | 284 |
| (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester | Fatty acid ethyl ester | 18.256 | 0.39 | C20H34O2 | 306 |
| Octadecanoic acid, ethyl ester | Fatty acid ethyl ester | 18.501 | 0.2 | C20H40O2 | 312 |
| Glycerol 1-palmitate | Monoacylglycerol | 21.486 | 1.2 | C19H38O4 | 330 |
| Coumarin | |||||
| 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 6.297 | 0.09 | C6H8O4 | 144 | |
| 3,5-dihydroxy-2-methyl-4H-pyran-4-one | 6.839 | 0.35 | C6H6O4 | 142 | |
| Phenol | |||||
| Salicylic acid | Phenolic acid | 8.335 | 0.55 | C7H6O3 | 138 |
| 2,4-bis(1,1-dimethylethyl)-phenol | Phenol | 11.117 | 1.17 | C14H22O | 206 |
| 4-hydroxy-3-methoxy-benzoic acid | Phenolic acid | 11.681 | 0.15 | C8H8O4 | 168 |
| Vitamin E | Tocochromanol | 26.323 | 1.63 | C29H50O2 | 430 |
| Organic acid | |||||
| 5-(hydroxymethyl)-2-furancarboxylic acid | Furoic acid | 9.953 | 1.05 | C6H6O4 | 142 |
| Tetradecanoic acid | Saturated fatty acid | 13.894 | 0.2 | C14H28O2 | 228 |
| n-Hexadecanoic acid | Saturated fatty acid | 15.994 | 24.13 | C16H32O2 | 256 |
| 9,12-octadecadienoic acid | Unsaturated fatty acid | 17.842 | 0.33 | C18H32O2 | 280 |
| (Z,Z,Z)-9,12,15-octadecatrienoic acid | Unsaturated fatty acid | 17.922 | 4.6 | C18H30O2 | 278 |
| Octadecanoic acid | Saturated fatty acid | 18.142 | 4.09 | C18H36O2 | 284 |
| Amide | |||||
| N,N-diethyl-4-methyl-benzamide | 11.988 | 0.07 | C12H17NO | 191 | |
| Hexadecanamide | 18.36 | 1.24 | C16H33NO | 255 | |
| Phytosterol | |||||
| (3β)-Stigmasta-5,24(28)-dien-3-ol | 28.046 | 4.31 | C29H48O | 412 | |
| Stigmasterol | 28.681 | 6.88 | C29H48O | 412 | |
| Terpenoid | |||||
| 5-hydroxy-4,7,7-trimethyl-bicyclo[2,2,1]heptan-2-one | 14.32 | 0.36 | C10H16O2 | 168 |
| No. | Compounds/Phytochemical Constituents | Binding Energy (kcal/mol) | Hydrogen Bonding | |
|---|---|---|---|---|
| Number | Amino Acid Interaction | |||
| Alizarin (positive control) | −8.63 | 2 | A:SER116:HN–UNK0:O4 :UNK0:H25–:UNK0:O2 | |
| Purpurin (positive control) | −8.84 | 4 | :UNK0:H25–A:ASN255:O :UNK0:H26–:UNK0:O4 :UNK0:H27–A:SER116:OG A:ASP313:CA–:UNK0:O2 | |
| 1 | 1,2-cyclopentanedione | −4.50 | 2 | A:SER116:HN–:UNK0:O2 A:ASN255:CA–:UNK0:O1 |
| 2 | d-galactonic acid, γ-lactone | −4.36 | 6 | A:SER116:HN–:UNK0:O5 :UNK0:H22–A:ASN255:OD1 :UNK0:H21–A:ASN255:OD1 :UNK0:H20–A:ASP313:O A:GLY316:CA–:UNK0:O3 :UNK0:C12–A:ASN255:O |
| 3 | Glycerin | −2.61 | 5 | A:SER116:HN–:UNK0:O3 :UNK0:H12–A:ASN255:OD1 :UNK0:H14–A:ASN255:OD1 :UNK0:H13–A:ASN255:OD1 :UNK0:C6–A:SER116:OG |
| 4 | Phytol | −8.31 | 1 | :UNK0:H61–A:ASP320:OD1 |
| 5 | Benzeneacetaldehyde | −5.30 | 1 | A:SER116:HN–:UNK0:O1 |
| 6 | Ethyl hydrogen malonate | −2.86 | 4 | A:SER116:HN–:UNK0:O4 :UNK0:H17–A:ASN255:O :UNK0:H17–:UNK0:O2 A:ASN255:CA–:UNK0:O2 |
| 7 | Hexadecanoic acid, methyl ester | −6.47 | 0 | - |
| 8 | Hexadecanoic acid, ethyl ester | −6.61 | 0 | - |
| 9 | (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester | −7.90 | 1 | A:SER122:HG–:UNK0:O2 |
| 10 | Octadecanoic acid, ethyl ester | −6.52 | 0 | - |
| 11 | Glycerol 1-palmitate | −5.86 | 4 | A:SER116:HN–:UNK0:O4 :UNK0:H60–A:LEU312:O :UNK0:H61–A:ASN255:OD1 A:GLY316:CA–:UNK0:O2 |
| 12 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | −5.16 | 3 | A:SER116:HN–:UNK0:O4 :UNK0:H17–A:ASN255:OD1 :UNK0:H18–A:SER116:OG |
| 13 | 3,5-dihydroxy-2-methyl-4H-pyran-4-one | −4.86 | 4 | A:SER116:HN–:UNK0:O4 :UNK0:H15–A:SER116:OG :UNK0:H16–A:ASN255:OD1 A:GLY316:CA–:UNK0:O1 |
| 14 | Salicylic acid | −4.42 | 3 | A:SER116:HN–:UNK0:O2 :UNK0:H15–A:SER116:OG :UNK0:H16–A:SER116:OG |
| 15 | 2,4-bis(1,1-dimethylethyl)-phenol | −7.03 | 0 | - |
| 16 | 4-hydroxy-3-methoxy-benzoic acid | −4.93 | 4 | A:SER116:HN–:UNK0:O2 :UNK0:H20–A:LEU312:O :UNK0:H19–A:ASN255:OD1 A:ASN255:CA–:UNK0:O1 |
| 17 | Vitamin E | −8.91 | 1 | :UNK0:H81–A:ASN222:OD1 |
| 18 | 5-(hydroxymethyl)-2-furancarboxylic acid | −3.63 | 2 | A:SER116:HN–:UNK0:O3 :UNK0:H16–A:ASN255:OD1 |
| 19 | Tetradecanoic acid | −5.41 | 1 | :UNK0:H44–A:ASP313:O |
| 20 | n-Hexadecanoic acid (Palmitic Acid) | −5.63 | 1 | :UNK0:H50–A:ASP313:O |
| 21 | 9,12-octadecadienoic acid | −6.12 | 2 | A:THR497:HN–:UNK0:O2 A:GLY225:CA–:UNK0:O2 |
| 22 | (Z,Z,Z)-9,12,15-octadecatrienoic acid | −6.76 | 0 | - |
| 23 | Octadecanoic acid | −5.93 | 3 | A:SER116:HN–:UNK0:O2 :UNK0:H56–A:ASN255:O :UNK0:H56–A:ASN255:OD1 |
| 24 | N,N-diethyl-4-methyl-benzamide | −6.81 | 1 | :UNK0:C3–A:GLY316:O |
| 25 | Hexadecanamide | −6.83 | 1 | :UNK0:H51–A:ASP320:OD1 |
| 26 | (3β)-Stigmasta-5,24(28)-dien-3-ol | −4.73 | 1 | :UNK0:H67–A:SER116:OG |
| 27 | Stigmasterol | −8.97 | 0 | - |
| 28 | 5-hydroxy-4,7,7-trimethyl-bicyclo[2,2,1]heptan-2-one | −5.72 | 1 | :UNK0:H28–A:ASN255:OD1 |
| No. | Compound | Binding Energy (kcal/mol) | Hydrogen Bonding | |
|---|---|---|---|---|
| Number | Amino Acid Interaction | |||
| Alizarin (positive control) | −8.09 | 2 | :UNK0:H25–A:ASP313:OD1 :UNK0:H26–A:ASP313:OD1 | |
| Purpurin (positive control) | −7.84 | 3 | A:THR124:HG1–:UNK0:O4 :UNK0:H26–:UNK0:O4 :UNK0:H27–A:ASP320:OD1 | |
| 1 | 1,2-cyclopentanedione | −4.15 | 3 | A:THR124:HG1–:UNK0:O2 A:GLY316:HN–:UNK0:O1 A:ALA317:HN–:UNK0:O1 |
| 2 | d-galactonic acid, γ-lactone | −3.71 | 5 | A:THR124:HG1–:UNK0:O3 A:ALA317:HN–:UNK0:O2 :UNK0:H21–A:ASN312:O :UNK0:H19–A:ASN312:O :UNK0:H20–A:ASP313:O |
| 3 | Glycerin | −2.53 | 6 | A:THR124:HG1–:UNK0:O2 A:ALA317:HN–:UNK0:O1 :UNK0:H12–A:ASN312:O :UNK0:H14–A:ASN312:O :UNK0:H13–A:ASP313:O :UNK0:H13–:UNK0:O1 |
| 4 | Phytol | −6.82 | 1 | :UNK0:H61–A:GLY316:O |
| 5 | Benzeneacetaldehyde | −4.77 | 2 | A:THR124:HG1–:UNK0:O1 :UNK0:C9–A:ASP313:O |
| 6 | Ethyl hydrogen malonate | −3.05 | 3 | A:ALA317:HN–:UNK0:O2 :UNK0:H17–A:ASP313:O :UNK0:H17–:UNK0:O2 |
| 7 | Hexadecanoic acid, methyl ester | −6.00 | 0 | - |
| 8 | Hexadecanoic acid, ethyl ester | −6.41 | 0 | - |
| 9 | (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester | −6.64 | 0 | - |
| 10 | Octadecanoic acid, ethyl ester | −6.42 | 1 | :UNK0:C21–A:ASP313:OD1 |
| 11 | Glycerol 1-palmitate | −4.89 | 0 | - |
| 12 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | −4.15 | 3 | A:SER122:HG–:UNK0:O2 A:THR124:HG1–:UNK0:O4 :UNK0:H17–A:ASP313:OD1 |
| 13 | 3,5-dihydroxy-2-methyl-4H-pyran-4-one | −3.91 | 2 | A:THR124:HG1–:UNK0:O4 :UNK0:H15–A:ASP313:OD1 |
| 14 | Salicylic acid | −4.46 | 3 | A:SER122:HG–:UNK0:O1 :UNK0:H15–A:ASP313:OD1 :UNK0:H16–A:ASP313:OD1 |
| 15 | 2,4-bis(1,1-dimethylethyl)-phenol | −7.11 | 0 | - |
| 16 | 4-hydroxy-3-methoxy-benzoic acid | −4.57 | 2 | :UNK0:H20–A:ASP313:O A:GLY316:CA–:UNK0:O1 |
| 17 | Vitamin E | −8.65 | 0 | - |
| 18 | 5-(hydroxymethyl)-2-furancarboxylic acid | −3.49 | 2 | A:THR124:HG1–:UNK0:O3 :UNK0:H16–A:ASP313:O |
| 19 | Tetradecanoic acid | −5.37 | 2 | A:THR124:HG1–:UNK0:O1 :UNK0:H44–A:ASP313:O |
| 20 | n-Hexadecanoic acid (Palmitic Acid) | −5.34 | 0 | - |
| 21 | 9,12-octadecadienoic acid | −6.22 | 1 | A:THR124:HG1–:UNK0:O2 |
| 22 | (Z,Z,Z)-9,12,15-octadecatrienoic acid | −6.06 | 0 | - |
| 23 | Octadecanoic acid | −5.48 | 0 | - |
| 24 | N,N-diethyl-4-methyl-benzamide | −6.39 | 0 | - |
| 25 | Hexadecanamide | −6.23 | 2 | :UNK0:H50–A:ASP313:OD1 A:SER122:CB–:UNK0:O1 |
| 26 | (3β)-Stigmasta-5,24(28)-dien-3-ol | −7.61 | 0 | - |
| 27 | Stigmasterol | −7.82 | 0 | - |
| 28 | 5-hydroxy-4,7,7-trimethyl-bicyclo[2,2,1]heptan-2-one | −5.78 | 1 | :UNK0:H28–A:ASP313:OD1 |
| No. | Compound | Binding Energy (kcal/mol) | Hydrogen Bonding | |
|---|---|---|---|---|
| Number | Amino Acid Interaction | |||
| Alizarin (positive control) | −8.17 | 3 | :UNK0:H25–A:GLY329:O :UNK0:H25–:UNK0:O2 :UNK0:H26–A:ASN228:OD1 | |
| Purpurin (positive control) | −8.45 | 5 | :UNK0:H25–A:GLY329:O :UNK0:H25–:UNK0:O3 :UNK0:H26–A:ASN265:OD1 :UNK0:H26–:UNK0:O4 :UNK0:H27–A:ASN228:OD1 | |
| 1 | 1,2-cyclopentanedione | −4.29 | 2 | A:SER127:HN–:UNK0:O2 A:ASN265:CA–:UNK0:O1 |
| 2 | d-galactonic acid, γ-lactone | −4.24 | 6 | A:SER127:HN–:UNK0:O5 :UNK0:H22–A:ASN265:OD1 :UNK0:H21–A:ASN265:OD1 :UNK0:H19–:UNK0:O4 :UNK0:H20–A:ASN228:OD1 :UNK0:H19–A:PHE231 |
| 3 | Glycerin | −2.38 | 5 | A:SER127:HN–:UNK0:O3 :UNK0:H12–A:ASN265:OD1 :UNK0:H14–A:ASN265:OD1 :UNK0:H13–A:ASN265:OD1 :UNK0:C6–A:SER127:OG |
| 4 | Phytol | −7.13 | 1 | :UNK0:H61–A:ASN228:O |
| 5 | Benzeneacetaldehyde | −4.95 | 1 | A:GLN332:HE21–:UNK0:O1 |
| 6 | Ethyl hydrogen malonate | −2.65 | 3 | A:SER127:HN–:UNK0:O4 :UNK0:H17–A:ASN265:OD1 :UNK0:H17–:UNK0:O1 |
| 7 | Hexadecanoic acid, methyl ester | −5.69 | 0 | - |
| 8 | Hexadecanoic acid, ethyl ester | −6.18 | 1 | A:GLN332:HE21–:UNK0:O1 |
| 9 | (Z,Z,Z)-9,12,15-octadecatrienoic acid, ethyl ester | −6.87 | 2 | A:GLN332:HE21–:UNK0:O1 :UNK0:C15–A:ASN228:OD1 |
| 10 | Octadecanoic acid, ethyl ester | −5.80 | 0 | - |
| 11 | Glycerol 1-palmitate | −5.24 | 4 | A:SER127:HN–:UNK0:O4 :UNK0:H60–:UNK0:O2 :UNK0:H61–A:ASN265:OD1 A:GLY329:CA–:UNK0:O1 |
| 12 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | −4.89 | 3 | A:SER127:HN–:UNK0:O4 :UNK0:H17–A:ASN265:OD1 :UNK0:H18–A:SER127:OG |
| 13 | 3,5-dihydroxy-2-methyl-4H-pyran-4-one | −4.60 | 3 | A:GLN332:HE21–:UNK0:O4 :UNK0:H15–A:ASN228:OD1 :UNK0:H16–A:ASP333:OD2 |
| 14 | Salicylic acid | −4.22 | 3 | A:SER127:HN–:UNK0:O3 :UNK0:H15–A:ASN265:OD1 :UNK0:H16–A:ASN265:OD1 |
| 15 | 2,4-bis(1,1-dimethylethyl)-phenol | −6.93 | 1 | :UNK0:H37–A:ASP326:OD2 |
| 16 | 4-hydroxy-3-methoxy-benzoic acid | −4.43 | 3 | A:SER127:HN–:UNK0:O4 :UNK0:H17–A:ASN265:OD1 :UNK0:H18–A:SER127:OG |
| 17 | Vitamin E | −7.15 | 1 | :UNK0:H81–A:SER127:OG |
| 18 | 5-(hydroxymethyl)-2-furancarboxylic acid | −3.44 | 2 | :UNK0:H16–A:ASP326:O :UNK0:H15–A:GLY329:O |
| 19 | Tetradecanoic acid | −4.81 | 0 | - |
| 20 | n-Hexadecanoic acid (Palmitic Acid) | −5.15 | 0 | - |
| 21 | 9,12-octadecadienoic acid | −6.20 | 0 | - |
| 22 | (Z,Z,Z)-9,12,15-octadecatrienoic acid | −5.76 | 0 | - |
| 23 | Octadecanoic acid | −5.33 | 2 | A:SER127:HN–:UNK0:O1 :UNK0:H56–A:ASN265:O |
| 24 | N,N-diethyl-4-methyl-benzamide | −6.54 | 1 | A:GLN332:HE21–:UNK0:O1 |
| 25 | Hexadecanamide | −6.03 | 2 | :UNK0:H50–A:ALA330:O :UNK0:H51–A:ASP333:OD2 |
| 26 | (3β)-Stigmasta-5,24(28)-dien-3-ol | −3.05 | 1 | :UNK0:C22–A:ASN265:OD1 |
| 27 | Stigmasterol | −8.06 | 0 | - |
| 28 | 5-hydroxy-4,7,7-trimethyl-bicyclo[2,2,1]heptan-2-one | −4.19 | 1 | A:SER127:HN–:UNK0:O2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. https://doi.org/10.3390/nu13072368
Pattarachotanant N, Prasansuklab A, Tencomnao T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients. 2021; 13(7):2368. https://doi.org/10.3390/nu13072368
Chicago/Turabian StylePattarachotanant, Nattaporn, Anchalee Prasansuklab, and Tewin Tencomnao. 2021. "Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E" Nutrients 13, no. 7: 2368. https://doi.org/10.3390/nu13072368
APA StylePattarachotanant, N., Prasansuklab, A., & Tencomnao, T. (2021). Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients, 13(7), 2368. https://doi.org/10.3390/nu13072368