Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Preparation of the Lupin Protein Concentrate (LPC)
2.3. Chemical Characterization of LPC
2.4. Antioxidant Activity of LPC
2.5. In Vivo Mouse Model of TNBS-Induced Colitis
2.5.1. Disease Activity Index (DAI)
2.5.2. Total Gelatinolytic Activity
2.6. Cookie Preparation
2.6.1. Savory Cookies
2.6.2. Sweet Cookies
2.7. In Vitro Colon Cancer Cell Assays
2.7.1. Testing LPC Bioactivity in Cookies
2.7.2. Wound Healing Assay
2.8. In Vivo Assays Using an AA-Induced Colitis Model
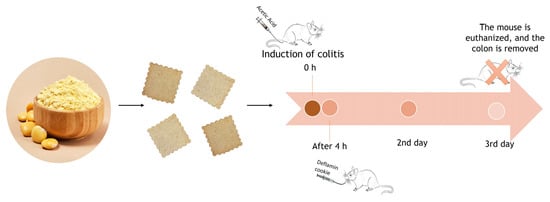
2.8.1. Colitis Induction and Experimental Groups
2.8.2. Anal Sphincter Pressure Measurement
2.8.3. Lipoperoxidation
2.8.4. Activity of Antioxidant Enzymes
2.8.5. Alkaline Comet Assay
2.8.6. Histopathological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Lupin Protein Concentrate (LPC) Holds Nutritional Value and Antioxidant Potential
3.2. LPC Reduces the Clinical Characteristics of Ulcerative Colitis, in a Dose-Dependent Manner
3.3. LPC Bioactivity, When Used as a Food Additive, Is Influenced by the Presence of Sugar
3.4. LPC-Enriched Cookies Are Effective as Functional Foods against AA-Induced Colitis In Vivo
3.5. Considerations on the LPC Cookies as Functional Foods
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, R.F.S.; Martins, J.T.; Duarte, C.M.M.; Vicente, A.A.; Pinheiro, A.C. Advances in nutraceutical delivery systems: From formulation design for bioavailability enhancement to efficacy and safety evaluation. Trends Food Sci. Technol. 2018, 78, 270–291. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Robles, H.; Castro-Ochoa, K.F.; Citalán-Madrid, A.F.; Schnoor, M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J. Gastroenterol. 2019, 25, 4181–4198. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Liguori, G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: A narrative review. Dig. Liver Dis. 2021, 53, 803–808. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Impact of diet on risk of IBD. Crohn’s Colitis 360 2020, 2, otz054. [Google Scholar] [CrossRef]
- Rogler, G.; Scharl, M.; Spalinger, M.; Yilmaz, B.; Zaugg, M.; Hersberger, M.; Schreiner, L.; Biedermann, L.; Herfarth, H. Diet and Inflammatory Bowel Disease: What Quality Standards Should Be Applied in Clinical and Laboratory Studies? Mol. Nutr. Food Res. 2021, 65, e2000514. [Google Scholar] [CrossRef]
- Mijan, M.A.; Lim, B.O. Diets, functional foods, and nutraceuticals as alternative therapies for inflammatory bowel disease: Present status and future trends. World J. Gastroenterol. 2018, 24, 2673–2685. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Piątek, D.; Filip, R. The influence of nutrients on inflammatory bowel diseases. J. Nutr. Metab. 2020, 2020, 2894169. [Google Scholar] [CrossRef] [Green Version]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [Green Version]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as therapeutic agents for inflammatory-related diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Banerjee, N.; Sirven, M.A.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1alpha axis in vivo and in vitro. J. Nutr. Biochem. 2017, 43, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, P.; Liu, J.; Gu, C.; Lu, X.; Li, Y.; Cao, Y.; Liu, B.; Fu, Y.; Zhang, N. In vivo study of the efficacy of the essential oil of Zanthoxylum bungeanum pericarp in dextran sulfate sodium-induced murine experimental colitis. J. Agric. Food Chem. 2017, 65, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Sahagún, M.; Gómez, M. Influence of protein source on characteristics and quality of gluten-free cookies. J. Food Sci. Technol. 2018, 55, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Direito, R.; Rocha, J.; Fernandes, J.; Sepodes, B.; Figueira, M.E.; Raymundo, A.; Lima, A.; Ferreira, R.B. Lupinus albus Protein Components Inhibit MMP-2 and MMP-9 Gelatinolytic Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 13286. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The Role of Extracellular Matrix Components in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Technological Potential of a Lupin Protein Concentrate as a Nutraceutical Delivery System in Baked Cookies. Foods 2021, 10, 1929. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef]
- Alemany-Cosme, E.; Sáez-González, E.; Moret, I.; Mateos, B.; Iborra, M.; Nos, P.; Sandoval, J.; Beltrán, B. Oxidative stress in the pathogenesis of Crohn’s disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants 2021, 10, 64. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Godínez-Méndez, L.A.; Gurrola-Díaz, C.M.; Zepeda-Nuño, J.S.; Vega-Magaña, N.; Lopez-Roa, R.I.; Íñiguez-Gutiérrez, L.; García-López, P.M.; Fafutis-Morris, M.; Delgado-Rizo, V. In Vivo Healthy Benefits of Galacto-Oligosaccharides from Lupinus albus (LAGOS) in Butyrate Production through Intestinal Microbiota. Biomolecules 2021, 11, 1658. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaneda, F.E.; Walia, B.; Vijay–Kumar, M.; Patel, N.R.; Roser, S.; Kolachala, V.L.; Rojas, M.; Wang, L.; Oprea, G.; Garg, P.; et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: Central role of epithelial-derived MMP. Gastroenterology 2005, 129, 1991–2008. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.A.; Balaraman, R.; Sailor, G.U.; Sen, D.B. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian J. Pharmacol. 2015, 47, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- Mota, J.; Lima, A.; Ferreira, R.B.; Raymundo, A. Lupin Seed Protein Extract Can Efficiently Enrich the Physical Properties of Cookies Prepared with Alternative Flours. Foods 2020, 9, 1064. [Google Scholar] [CrossRef]
- Hartmann, R.M.; Fillmann, H.S.; Morgan Martins, M.I.; Meurer, L.; Marroni, N.P. Boswellia serrata has beneficial anti-inflammatory and antioxidant properties in a model of experimental colitis. Phytother. Res. 2014, 28, 1392–1398. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Schemitt, E.G.; Da Silva, J.; Marroni, N.P.; Lima, A.; Ferreira, R.B. Combination of trans-Resveratrol and ε-Viniferin Induces a Hepatoprotective Effect in Rats with Severe Acute Liver Failure via Reduction of Oxidative Stress and MMP-9 Expression. Nutrients 2021, 13, 3677. [Google Scholar] [CrossRef]
- Vercelino, R.; Tieppo, J.; Dias, A.S.; Marroni, C.A.; Garcia, E.; Meurer, L.; Picada, J.N.; Marroni, N.P. N-Acetylcysteine effects on genotoxic and oxidative stress parameters in cirrhotic rats with hepatopulmonary syndrome. Basic Clin. Pharmacol. Toxicol. 2008, 102, 370–376. [Google Scholar] [CrossRef]
- Nadin, S.B.; Vargas-Roig, L.M.; Ciocca, D.R. A silver staining method for single-cell gel assay. J. Histochem. Cytochem. 2001, 49, 1183–1186. [Google Scholar] [CrossRef] [Green Version]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. J. Food Process. Preserv. 2020, 2, 6. [Google Scholar] [CrossRef]
- Siger, A.; Czubinski, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kalucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Direito, R.; Lima, A.; Rocha, J.; Ferreira, R.B.; Mota, J.; Rebelo, P.; Fernandes, A.; Pinto, R.; Alves, P.; Bronze, R.; et al. Dyospiros kaki phenolics inhibit colitis and colon cancer cell proliferation, but not gelatinase activities. J. Nutr. Biochem. 2017, 46, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118, 109351. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Lima, A.; Gonçalves, M.M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.; Ferreira, R.B.; et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Szwajgier, D.; Paduch, R.; Kukuła-Koch, W.; Polak-Berecka, M.; Waśko, A. Study on Biological Activity of Bread Enriched with Natural Polyphenols in Terms of Growth Inhibition of Tumor Intestine Cells. J. Med. Food 2020, 23, 181–190. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Kaszuba, K.; Ryszawy, D.; Czyż, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. Biomed. Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Dev. Ther. 2013, 7, 1341–1357. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos, J.; Araújo, O.; Goulart, M. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Tahan, G.; Gramignoli, R.; Marongiu, F.; Aktolga, S.; Cetinkaya, A.; Tahan, V.; Dorko, K. Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig. Dis. Sci. 2011, 56, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shifrin, H.; Nadler-Milbauer, M.; Shoham, S.; Weinstock, M. Rivastigmine alleviates experimentally induced colitis in mice and rats by acting at central and peripheral sites to modulate imunne responses. PLoS ONE 2013, 8, e576688. [Google Scholar] [CrossRef] [Green Version]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.P.; Mori, T.A.; Sipsas, S.; Barden, A.; Puddey, I.B.; Burke, V.; Hall, R.S.; Hodgson, J.M. Lupin-enriched bread increases satiety and reduces energy intake acutely. Am. J. Clin. Nutr. 2006, 84, 975–980. [Google Scholar] [CrossRef] [Green Version]
- Capraro, J.; Magni, C.; Scarafoni, A.; Caramanico, R.; Rossi, F.; Morlacchini, M.; Duranti, M. Pasta supplemented with isolated lupin protein fractions reduces body weight gain and food intake of rats and decreases plasma glucose concentration upon glucose overload trial. Food Funct. 2014, 5, 375–380. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Morales-Polanco, E.; Campos-Veja, R.; Gaytán-Martínez, M.; Enriquez, L.G.; Loarca-Piña, G. Functional and textural properties of a dehulled oat (Avena sativa L.) and pea (Pisum sativum) protein isolate cracker. LWT 2017, 86, 418–423. [Google Scholar] [CrossRef]




| Component | Amount |
|---|---|
| Moisture (g/100 g) | 10.31 ± 2.53 |
| Total Ash (g/100 g) | 7.25 ± 0.27 |
| Crude Fat (g/100 g) | 0.31 ± 0.05 |
| Crude Protein (g/100 g) | 20.09 ± 0.58 |
| Carbohydrates * (g/100 g) | 62.05 |
| Total Polyphenols (mg/100 g) | 35.19 ± 2.5 |
| Antioxidant Activity | |
| FRAP (mg AAE/10 mg LPC) | 351.19 ± 2.5 |
| DPPH (mg AAE/ 10 mg LPC) | 273.9 ± 2.03 |
| DAI Score | Colon Length (cm) | Total Gelatinolytic Activity (%) | |||
|---|---|---|---|---|---|
| Mean ± SD | Min; Max | Mean ± SD | Min; Max | Mean ± SD | |
| Co | 0 ± 0 | (0; 0) | 11.4 ± 0.4 | (10.9; 11.8) | 0 ± 1.9 |
| Col | 3.4 ± 0.4 | (2; 4) | 7.2 ± 0.77 # | (6.5; 8.2) | 100 ± 1.5 |
| Col + LPC (0.1 g/kg) | 2.5 ± 0.6 * | (1; 3) | 10. 8 ± 2.2 | (8.7; 12.8) | 83.1 ± 1.7 * |
| Col + LPC (1 g/kg) | 2.2 ± 0.3 * | (1; 3) | 9.7 ± 1.3 | (8.8; 10.9) | 71.6 ± 1.3 ** |
| Col + LPC (10 g/kg) | 1.5 ± 0.2 ** | (0; 3) | 10.4 ± 0.4 | (10.1; 10.9) | 65.4 ± 1.2 ** |
| COX-2 | TNF-α | |
|---|---|---|
| Co | 0.9 ± 0.3 * | 0.7 ± 0.1 * |
| Co + LPCc | 1.94 ± 1.5 * | 2.4 ± 0.6 *,# |
| Col | 11.7 ± 1.98 # | 8.7 ± 1.6 # |
| Col + Cc | 5.1 ± 2.1 *,# | 2.5 ± 0.6 *,# |
| Col + LPCc | 0.9 ± 0.2 * | 1.2 ± 0.2 *,# |
| Group | Damage Index | Damage Frequency |
|---|---|---|
| Co | 17.0 ± 5.7 # | 17.0 ± 5.7 # |
| Co + LPCc | 21.4 ± 3.6 # | 21.4 ± 3.6 # |
| Col | 68.8 ± 7.9 * | 57.5 ± 11.5 * |
| Col + Cc | 40.3 ± 9.2 *# | 35.3 ± 10.4 *# |
| Col + LPCc | 17.4 ± 3.4 # | 17.2 ± 3.3 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, J.; Casimiro, S.; Fernandes, J.; Hartmann, R.M.; Schemitt, E.; Picada, J.; Costa, L.; Marroni, N.; Raymundo, A.; Lima, A.; et al. Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients 2022, 14, 2102. https://doi.org/10.3390/nu14102102
Mota J, Casimiro S, Fernandes J, Hartmann RM, Schemitt E, Picada J, Costa L, Marroni N, Raymundo A, Lima A, et al. Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients. 2022; 14(10):2102. https://doi.org/10.3390/nu14102102
Chicago/Turabian StyleMota, Joana, Sandra Casimiro, João Fernandes, Renata M. Hartmann, Elizângela Schemitt, Jaqueline Picada, Luís Costa, Norma Marroni, Anabela Raymundo, Ana Lima, and et al. 2022. "Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress" Nutrients 14, no. 10: 2102. https://doi.org/10.3390/nu14102102
APA StyleMota, J., Casimiro, S., Fernandes, J., Hartmann, R. M., Schemitt, E., Picada, J., Costa, L., Marroni, N., Raymundo, A., Lima, A., & Ferreira, R. B. (2022). Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients, 14(10), 2102. https://doi.org/10.3390/nu14102102