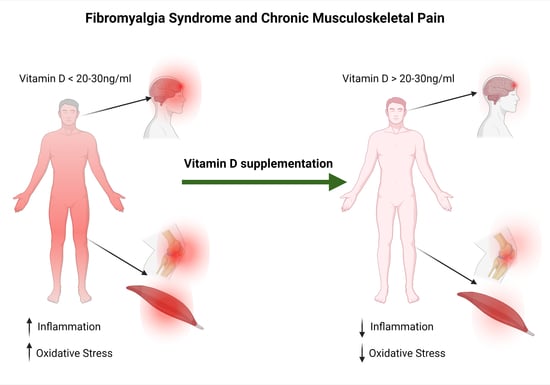
The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Quality Assessment
3. Results
3.1. Eligibility
3.2. VAS Scale
3.3. Other Pain Scales
3.4. Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2596 people with fibromyalgia. BMC Muscul. Disord. 2007, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The prevalence of fibromyalgia in the general population: A comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Häuser, W.; Ablin, J.; Fitzcharles, M.A.; Littlejohn, G.; Luciano, J.V.; Usui, C.; Walitt, B. Fibromyalgia. Nat. Rev. Dis. Prim. 2015, 1, 15022. [Google Scholar] [CrossRef]
- D′Aoust, R.F.; Rossiter, A.G.; Elliott, A.; Ji, M.; Lengacher, C.; Groer, M. Women Veterans, a Population at Risk for Fibromyalgia: The Associations Between Fibromyalgia, Symptoms, and Quality of Life. Mil. Med. 2017, 182, e1828–e1835. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, K.M. Sleep disturbances linked to fibromyalgia. Holist. Nurs. Pract. 2003, 17, 120–127. [Google Scholar] [CrossRef]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [Green Version]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K. European Network on ME/CFS (EUROMENE). Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.P.; Ferreira, E.A.; Matsutani, L.A.; Pereira, C.A.; Assumpção, A. Quantifying pain threshold and quality of life of fibromyalgia patients. Clin. Rheumatol. 2005, 24, 266–271. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Semin. Arthritis Rheum. 2021, 51, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.; Marshall, T. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering with Host Metabolism, Gene Expression, and Immunity. Front. Pediatr. 2018, 6, 373. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, J.; Hurley, J.A. Fibromyalgia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 13 October 2021. [Google Scholar]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.; Schweinhardt, P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res. Treat. 2012, 2012, 741746. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Das, S.K.; Mahdi, A.A. Oxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: Implications in the pathogenesis of the disease. Clin. Exp. Rheumatol. 2013, 31 (Suppl. 79), S128–S133. [Google Scholar] [PubMed]
- Batista, E.D.; Andretta, A.; de Miranda, R.C.; Nehring, J.; Dos Santos Paiva, E.; Schieferdecker, M.E. Food intake assessment and quality of life in women with fibromyalgia. Rev. Bras. Reumatol. Engl. Ed. 2016, 56, 105–110. [Google Scholar] [CrossRef]
- Joustra, M.L.; Minovic, I.; Janssens, K.A.M.; Bakker, S.J.L.; Rosmalen, J.G.M. Vitamin and mineral status in chronic fatigue syndrome and fibromyalgia syndrome: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0176631. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Vitamin D and Muscle Sarcopenia in Aging. Methods Mol. Biol. 2020, 2138, 29–47. [Google Scholar] [CrossRef]
- Ellis, S.D.; Kelly, S.T.; Shurlock, J.H.; Hepburn, A.L.N. The role of vitamin D testing and replacement in fibromyalgia: A systematic literature review. BMC Rheumatol. 2018, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caprio, M.; Mammi, C.; Rosano, G.M. Vitamin D: A novel player in endothelial function and dysfunction. Arch. Med. Sci. 2012, 8, 4–5. [Google Scholar] [CrossRef]
- Shipton, E.A.; Shipton, E.E. Vitamin D and Pain: Vitamin D and Its Role in the Aetiology and Maintenance of Chronic Pain States and Associated Comorbidities. Pain Res. Treat. 2015, 2015, 904967. [Google Scholar] [CrossRef] [Green Version]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Patnode, C.D.; Berkman, N.D.; Bass, E.B.; Chang, S.; Hartling, L.; Murad, M.H.; Treadwell, J.R.; Kane, R.L. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK91433/ (accessed on 8 March 2012).
- Warner, A.E.; Arnspiger, S.A. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J. Clin. Rheumatol. 2008, 14, 12–16. [Google Scholar] [CrossRef]
- Sakalli, H.; Arslan, D.; Yucel, A.E. The effect of oral and parenteral vitamin D supplementation in the elderly: A prospective, double-blinded, randomized, placebo-controlled study. Rheumatol. Int. 2012, 32, 2279–2283. [Google Scholar] [CrossRef]
- Schreuder, F.; Bernsen, R.M.; van der Wouden, J.C. Vitamin D supplementation for nonspecific musculoskeletal pain in non-Western immigrants: A randomized controlled trial. Ann. Fam. Med. 2012, 10, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Knutsen, K.V.; Madar, A.A.; Brekke, M.; Meyer, H.E.; Natvig, B.; Mdala, I.; Lagerlv, P. Effect of vitamin D on musculoskeletal pain and headache: A randomized, double-blind, placebo-controlled trial among adult ethnic minorities in Norway. Pain 2014, 155, 2591–2598. [Google Scholar] [CrossRef]
- Wepner, F.; Scheuer, R.; Schuetz-Wieser, B.; Machacek, P.; Pieler-Bruha, E.; Cross, H.S.; Hahne, J.; Friedrich, M. Effects of vitamin D on patients with fibromyalgia syndrome: A randomized placebo-controlled trial. Pain 2014, 155, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sandoughi, M.; Zakeri, Z.; Mirhosainee, Z.; Mohammadi, M.; Shahbakhsh, S. The effect of vitamin D on nonspecific low back pain. Int. J. Rheum. Dis. 2015, 18, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendelman, O.; Itzhaki, D.; Makarov, S.; Bennun, M.; Amital, H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus 2015, 24, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Zabihiyeganeh, M.; Jahed, S.A.; Khiabani, E.; Nojomi, M.; Ghaffari, S. Effects of vitamin D optimization on quality of life of patients with fibromyalgia: A randomized controlled trial. Med. J. Islam. Repub. Iran 2018, 32, 29. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Camargo, C.A., Jr.; Malihi, Z.; Bartley, J.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Scragg, R. Monthly vitamin D supplementation, pain, and pattern of analgesic prescription: Secondary analysis from the randomized, double-blind, placebo-controlled Vitamin D Assessment study. Pain 2018, 159, 1074–1082. [Google Scholar] [CrossRef]
- Aldaoseri, H.A.; Zubairi, M.B. Vitamin D deficiency and treatment in Iraqi patients with primary fibromyalgia syndrome. Egypt. Rheumatol. 2020, 42, 47–50. [Google Scholar] [CrossRef]
- Schlögl, M.; Chocano-Bedoya, P.; Dawson-Hughes, B.; Orav, E.J.; Freystaetter, G.; Theiler, R.; Kressig, R.W.; Egli, A.; Bischoff-Ferrari, H.A. Effect of Monthly Vitamin D on Chronic Pain Among Community-Dwelling Seniors: A Randomized, Double-Blind Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 356–361. [Google Scholar] [CrossRef]
- Abdeen, H.A.A.; Rodriguez-Sanz, D.; Ewidea, M.; Al-Hamaky, D.M.A.; Mohamed, M.A.E.; Elerian, A.E. Efficacy of Vitamin D Supplementation in Addition to Aerobic Exercise Training in Obese Women with Perceived Myalgia: A Single-Blinded Randomized Controlled Clinical Trial. Nutrients 2021, 13, 1819. [Google Scholar] [CrossRef]
- Ali, M.; Uddin, Z.; Hossain, A. Combined Effect of Vitamin D Supplementation and Physiotherapy on Reducing Pain Among Adult Patients with Musculoskeletal Disorders: A Quasi-Experimental Clinical Trial. Front. Nutr. 2021, 8, 717473. [Google Scholar] [CrossRef]
- Lozano-Plata, L.I.; Vega-Morales, D.; Esquivel-Valerio, J.A.; Garza-Elizondo, M.A.; Galarza-Delgado, D.A.; Silva-Luna, K.; Serna-Peña, G.; Sifuentes-Ramírez, J.; Garza-Guerra, A.J.; Díaz-Niño de Rivera, R. Efficacy and safety of weekly vitamin D3 in patients with fibromyalgia: 12-week, double-blind, randomized, controlled placebo trial. Clin. Rheumatol. 2021, 40, 3257–3264. [Google Scholar] [CrossRef]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crofford, L.J. Chronic Pain: Where the Body Meets the Brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167–183. [Google Scholar] [PubMed]
- Volker, G.; van Vree, F.; Wolterbeek, R.; van Gestel, M.; Smeets, R.; Köke, A.; Vlieland, T.V. Long-Term Outcomes of Multidisciplinary Rehabilitation for Chronic Musculoskeletal Pain. Musculoskelet. Care 2017, 15, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.P.; German, J.S.; Samet, J.H.; Cheng, D.M.; Lloyd-Travaglini, C.A.; Saitz, R. Primary Care Patients with Drug Use Report Chronic Pain and Self-Medicate with Alcohol and Other Drugs. J. Gen. Intern. Med. 2016, 31, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, K.I.; Obata, H. Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int. J. Mol. Sci. 2019, 20, 822. [Google Scholar] [CrossRef] [Green Version]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [Green Version]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; MacDermid, J.C.; Walton, D.M.; Richardson, J. Chronic pain self-management support with pain science education and exercise (COMMENCE): Study protocol for a randomized controlled trial. Trials 2015, 16, 462. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.; Bradnam, L.; Barr, C. The relationship between knowledge of pain neurophysiology and fear avoidance in people with chronic pain: A point in time, observational study. Physiother. Theory Pract. 2016, 32, 271–276. [Google Scholar] [CrossRef]
- Ablin, J.; Fitzcharles, M.A.; Buskila, D.; Shir, Y.; Sommer, C.; Häuser, W. Treatment of fibromyalgia syndrome: Recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid. Based Complement. Altern. Med. 2013, 2013, 485272. [Google Scholar] [CrossRef] [Green Version]
- Benson, J.; Wilson, A.; Stocks, N.; Moulding, N. Muscle pain as an indicator of vitamin D deficiency in an urban Australian Aboriginal population. Med. J. Aust. 2006, 185, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, A.; Abdel-Nasser, A.M.; Hamdy, A.; Omran, A.A.; El-Rehany, M.A. Hypovitaminosis D in female patients with chronic low back pain. Clin. Rheumatol. 2007, 26, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Atherton, K.; Berry, D.J.; Parsons, T.; Macfarlane, G.J.; Power, C.; Hyppönen, E. Vitamin D and chronic widespread pain in a white middle-aged British population: Evidence from a cross-sectional population survey. Ann. Rheum. Dis. 2009, 68, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Shirvani, J.S.; Firouzjahi, A.; Heidari, P.; Hajian-Tilaki, K.O. Association between nonspecific skeletal pain and vitamin D deficiency. Int. J. Rheum. Dis. 2010, 13, 340–346. [Google Scholar] [CrossRef]
- Knutsen, K.V.; Brekke, M.; Gjelstad, S.; Lagerløv, P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: A cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand. J. Prim. Health Care 2010, 28, 166–171. [Google Scholar] [CrossRef] [Green Version]
- McBeth, J.; Pye, S.; O’Neill, T.; Macfarlane, G.; Tajar, A.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.; Finn, J.D.; et al. Musculoskeletal pain is associated with very low levels of vitamin D in men: Results from the European Male Ageing Study. Ann. Rheum. Dis. 2010, 69, 1448–1452. [Google Scholar] [CrossRef] [Green Version]
- Straube, S.; Derry, S.; Straube, C.; Moore, R.A. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst. Rev. 2015, 2015, CD007771. [Google Scholar] [CrossRef]
- Dogru, A.; Balkarli, A.; Cobankara, V.; Tunc, S.E.; Sahin, M. Effects of Vitamin D Therapy on Quality of Life in Patients with Fibromyalgia. Eurasian J. Med. 2017, 49, 113–117. [Google Scholar] [CrossRef]
- Helde-Frankling, M.; Björkhem-Bergman, L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017, 18, 2170. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.M.; Nagi, K.; Thillaiappan, N.B.; Sukumaran, V.; Akhtar, S. Vitamin D and Its Potential Interplay with Pain Signaling Pathways. Front. Immunol. 2020, 11, 820. [Google Scholar] [CrossRef]
- Liu, X.; Nelson, A.; Wang, X.; Farid, M.; Gunji, Y.; Ikari, J.; Iwasawa, S.; Basma, H.; Feghali-Bostwick, C.; Rennard, S.I. Vitamin D modulates prostaglandin E2 synthesis and degradation in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2014, 50, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmi, V.; Bellia, A.; Gentileschi, P.; Lombardo, M.; D’Adamo, M.; Lauro, D.; Sbraccia, P. Parathyroid hormone in surgery-induced weight loss: No glucometabolic effects but potential adaptive response to skeletal loading. Endocrine 2018, 59, 288–295. [Google Scholar] [CrossRef]
- Gupta, N.; Farooqui, K.J.; Batra, C.M.; Marwaha, R.K.; Mithal, A. Effect of oral versus intramuscular Vitamin D replacement in apparently healthy adults with Vitamin D deficiency. Indian J. Endocrinol. Metab. 2017, 21, 131–136. [Google Scholar] [CrossRef]
- Khaw, K.T.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Camargo, C.A., Jr.; Scragg, R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: Secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017, 5, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Thacher, T.D.; Fischer, P.R.; Pettifor, J.M.; Lawson, J.O.; Isichei, C.O.; Chan, G.M. Case-control study of factors associated with nutritional rickets in Nigerian children. J. Pediatr. 2000, 137, 367–373. [Google Scholar] [CrossRef]
- Atapattu, N.; Shaw, N.; Högler, W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr. Res. 2013, 74, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Bouillon, R.; van Schoor, N.M.; Vanderschueren, D.; Verschueren, S.; Kuchuk, N.; Milisen, K.; Boonen, S. Reducing fracture risk with calcium and vitamin D. Clin. Endocrinol. 2010, 73, 277–285. [Google Scholar] [CrossRef]
- Forney, L.A.; Earnest, C.P.; Henagan, T.M.; Johnson, L.E.; Castleberry, T.J.; Stewart, L.K. Vitamin D status, body composition, and fitness measures in college-aged students. J. Strength Cond. Res. 2014, 28, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones 2016, 15, 471–488. [Google Scholar] [CrossRef] [Green Version]
- Wagatsuma, A.; Sakuma, K. Vitamin D signaling in myogenesis: Potential for treatment of sarcopenia. BioMed Res. Int. 2014, 2014, 121254. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.K.; Cesari, M.; Ferrucci, L.; Cherubini, A.; Maggio, D.; Bartali, B.; Johnson, M.A.; Schwartz, G.G.; Kritchevsky, S.B. Association between vitamin D status and physical performance: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kujach, S.; Lyzwinski, D.; Chroboczek, M.; Bialowas, D.; Antosiewicz, J.; Laskowski, R. The Effect of Vitamin D3 Supplementation on Physical Capacity among Active College-Aged Males. Nutrients 2020, 12, 1936. [Google Scholar] [CrossRef]
- Aschauer, R.; Unterberger, S.; Zöhrer, P.A.; Draxler, A.; Franzke, B.; Strasser, E.M.; Wagner, K.H.; Wessner, B. Effects of Vitamin D3 Supplementation and Resistance Training on 25-Hydroxyvitamin D Status and Functional Performance of Older Adults: A Randomized Placebo-Controlled Trial. Nutrients 2021, 14, 86. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]




| Sample Size | Age Mean | Age Mean | Vitamin D Level (ng/mL)-Baseline | Vitamin D Level (ng/mL)-End of Treatment | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. | First Author | Year | Country | Study Design | Topic | Gender | Season of Measurement | Duration (Weeks) | Control Characteristics | Intervention Group | Control | Intervention Group | Control | p-Value | Intervention Group | Control | Intervention Group | Control | Dosage (Weekly IU) | Threshold of Vit D Deficiency (ng/mL) | Method of Vit D Measure |
| [29] | Warner | 2008 | USA | RCT | CMP | F | ? | 12 | Diffuse pain/low 25OH Vit D | 20 | 22 | 58.0 ± 7.3 | 56.7 ± 11.3 | 0.634 | 16.8 ± 2.9 | 15.9 ± 3.6 | ? | ? | 50,000 | <20 | ? |
| [30] | Sakalli | 2012 | Turkey | RCT | CMP | M + F | Winter–spring | 4 | Placebo | 30 | 30 | 69.8 ± 3.7 | 68.9 ± 2.7 | ns | 20.9 ± 9.5 | 21.2 ± 7.4 | 27.0 ± 12.0 | 21.0 ± 5.5 | 300,000 | <12.5 | RIA |
| [31] | Scheuder | 2012 | Netherlands | RCT ç | CMP | M + F | ? | 12 | Placebo | 44 | 40 | 42.9 ± 9.5 | 40.8 ± 11.3 | ns | 19.7 ± 10.7 | 19.7 ± 8.8 | 20.2 ± 10.3 | 19.6 ± 9.0 | 25,000 | <20 | RIA |
| [32] | Knutsen | 2014 | Norway | RCT ° | CMP | M + F | Winter–spring | 16 | Placebo | 84/85 | 82 | 37.5 ± 8.1 | 38.0 ± 7.2 | ? | 28.7 ^ | 29.2 ^ | 48.8 ^ | 27.5 ^ | (1) 7000 (2) 2800 $ | ? | LC/MS/MS |
| [33] | Wepner | 2014 | Austria | RCT | FMS | M + F | Summer | 49 | Placebo | 15 | 15 | 49.1 ± 5.7 | 47.6 ± 4.9 | 0.438 | 19.0 ± 5.9 | 20.9 ± 6.3 | 26.3 ± 6.9 | 26.3 ± 11.7 | # | <32 | ? |
| [34] | Sandoughi | 2015 | Iran | RCT | CMP | M + F | Spring–autumn | 8 | Placebo | 26 | 27 | 33.2 ± 6.5 | 33.2 ± 6.5 | 0.430 | 17.9 ± 9.0 | 19.8 ± 9.6 | 27.5 ± 9.0 | 18.9 ± 7.8 | 50,000 | <20 | ELISA |
| [35] | Gendelman | 2015 | Israel | RCT | CMP | M + F | Autumn–spring | 12 | Placebo | 38 | 36 | 56.8 ± 13.1 | 57.3 ± 13.8 | 0.885 | 21.8 ± 8.7 | 25.5 ± 13.0 | 31.7 ± 22.8 | 24.0 ± 13.1 | 28,000 | <30 | RIA |
| [36] | Mirzaei | 2018 | Iran | RCT | FMS | M + F | ? | 8 | Antidepressant | 37 | 37 | 42.1 ± 10.8 | 41 ± 10.3 | ? | 11.4 ± 6.5 | 13.4 ± 7.3 | 33.5 ± 12.2 | 13.3 ± 7.2 | 50,000 | <20 | RIA |
| [37] | Wu | 2018 | New Zealand | RCT § | CMP | M + F | ? | 3.3 yrs | Placebo | 2558 | 2550 | 65.9 ± 8.3 | 65.9 ± 8.3 | ns | 26.6 ± 9.0 | 26.3 ± 9.0 | 54.1 ± 16.0 | 26.4 ± 11.6 | 25,000 | <20 | LC/MS/MS |
| [38] | ALdaoseri | 2019 | Iraq | RCT | FMS | M + F | Spring–autumn | 12 | Antidepressant | 53 | 53/54 | 34.3 ± 9.5 | 35.1 ± 11.6 | ? | ? | ? | ? | ? | 50,000 | <20 | HA |
| [39] | Schlögl | 2019 | Switzerland | RCT ° | CMP | M + F | Year | 52 | Vitamin D 800 IU/day | 66 | 67/67 | 77 ± 4.7 | 78 ± 5.3 | ? | 18.4 ± 7.6 | 20.9 ± 9.2 | ? | ? | (1) 14,000 (2) 2800 + 75 mg of calcifediol (3) 5600 & | <20 | LC/MS/MS |
| [40] | Abdeen | 2021 | Egypt | RCT ° | CMP | F | Winter–spring | 12 | Aerobic training | 15 | 15/15 | 34.8 ± 2.6 | 35.4 ± 2.7 | 0.830 | 17.1 ± 1.2 | 16.4 ± 1.7 | 23.9 ± 4.3 | 17.6 ± 2.3 | 50,000 | <10 | BioPlex |
| [41] | Ali | 2021 | Bangladesh | RCT | CMP | M + F | Year | 8 | Physiotherapy | 72 | 63 | 51.2 ± 13.3 | 0.065 | ? | ? | ? | ? | 50.000 | <20 | CMIA | |
| [42] | Lozano-Plata | 2021 | Mexico | RCT | FMS | F | ? | 12 | Placebo | 40 | 40 | 50.3 ± 11.9 | 51.4 ± 9.5 | ns | 20.1 ± 14.5 | 12.6 ± 13.4 | 51.1 | 20.8 | 50.000 | <20 | ELFA |
| VAS—Baseline | VAS—End of Treatment | Association between Low Vitamin D and MS Pain | Effects of Vitamin D Supplementation | Effects of Vitamin D Supplementation in People with Vitamin D Insufficiency at Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. | First Author | Case | Control | Case | Control | p-Value | |||
| [29] | Warner | 67.8 ± 22.0 | 67.3 ± 22.8 | 64.7 ± 18.0 | 53.6 ± 26.8 | 0.727 | = | = | = |
| [30] | Sakalli | 59 ± 24 | 59 ± 27 | 51 ± 23 | 55 ± 28 | < 0.01 | ↑ * | ||
| [31] | Scheuder | 60.6 ± 21.2 | 65.1 ± 17.2 | 65.2 ± 16.1 | 66.0 ± 17.1 | ns | ↑ @ | = | |
| [32] | Knutsen | 22.3 | 25.2 | 14 | 14.3 | 0.18 | = | = | |
| [33] | Wepner | 62.0 ± 20.3 | 55.2 ± 20.5 | 68.7 ± 12.5 | 55.2 ± 21.8 | 0.025 | ↑ | ↑ § | |
| [34] | Sandoughi | 54.2 ± 16.5 | 64.4 ± 16.2 | 30.3 ± 31.4 | 31.1 ± 30.8 | <0.001 | = | ||
| [35] | Gendelman | 72.2 ± 20.6 | 63.3 ± 22.9 | 48.6 ± 26.0 | 54.6 ± 28.3 | 0.612 | ↑ | ↑ | |
| [40] | Abdeen | 61 ± 8.9 | 64 ± 8 | 27.5 ± 12.5 | 30.3 ± 12.5 | 0.001 | ↑ # | ||
| [42] | Lozano-Plata | 60 ± 30 | 60 ± 35 | 49 | 51.9 | 0.705 | = | ||
| Ref. | First Author | Pain Scale | Association between Low Vitamin D and CMP Pain | Effects of Vitamin D Supplementation | Effects of Vitamin D Supplementation in People with Vitamin D Insufficiency at Baseline |
|---|---|---|---|---|---|
| [29] | Warner | FPS | = | ||
| [30] | Sakalli | BP | ↑ * | ||
| [31] | Scheuder | Likert scale | ↑ @ | ↑ @ | ↑ @ |
| [33] | Wepner | FIQ | = | ||
| [35] | Gendelman | MPQ | ↑ | = | |
| [36] | Mirzaei | WPI, FIQ | ↑ & | ||
| [37] | Wu | PIQ-6 | = | ↑ $ | |
| [38] | ALdaoseri | WPI, SSS | ↑ | ↑ | ↑ |
| [39] | Schlögl | MPQ | = | ↑ | |
| [41] | Ali | BPI | ↑ | ||
| [42] | Lozano-Plata | FIQ | = |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Feraco, A.; Ottaviani, M.; Rizzo, G.; Camajani, E.; Caprio, M.; Armani, A. The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients 2022, 14, 3010. https://doi.org/10.3390/nu14153010
Lombardo M, Feraco A, Ottaviani M, Rizzo G, Camajani E, Caprio M, Armani A. The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients. 2022; 14(15):3010. https://doi.org/10.3390/nu14153010
Chicago/Turabian StyleLombardo, Mauro, Alessandra Feraco, Morena Ottaviani, Gianluca Rizzo, Elisabetta Camajani, Massimiliano Caprio, and Andrea Armani. 2022. "The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain" Nutrients 14, no. 15: 3010. https://doi.org/10.3390/nu14153010
APA StyleLombardo, M., Feraco, A., Ottaviani, M., Rizzo, G., Camajani, E., Caprio, M., & Armani, A. (2022). The Efficacy of Vitamin D Supplementation in the Treatment of Fibromyalgia Syndrome and Chronic Musculoskeletal Pain. Nutrients, 14(15), 3010. https://doi.org/10.3390/nu14153010