Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Recruitment for the Clinical Trial
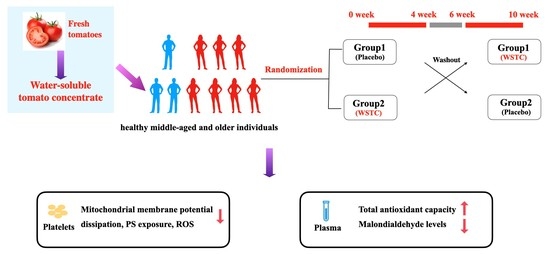
2.2. Study Design
2.3. Supplement Preparation
2.4. Sample Size Planning for the Clinical Trial
2.5. Basic Information and Anthropometric Measurement
2.6. Laboratory Measurement
2.7. Detection of Platelet Aggregation and Activation
2.8. Measurement of ROS, ΔΨm and PS Exposure in Human Platelets
2.9. Statistical Analysis
3. Results
3.1. Subsection
3.2. Effects of WSTC Supplementation on TAC and MDA in Healthy Middle-Aged and Elderly Adults
3.3. WSTC Supplementation Attenuated Platelet ROS Generation in Healthy Middle-Aged and Elderly Adults
3.4. WSTC Supplementation Attenuated Platelet ΔΨm Dissipation and PS Exposure in Healthy Middle-Aged and Elderly Adults
3.5. WSTC Supplementation Attenuated ROS Generation and ΔΨm Dissipation in Thrombin-Treated Platelets
3.6. WSTC Supplementation Inhibited Platelet Aggregation and Activation in Healthy Middle-Aged and Elderly Adults
3.7. Safety Evaluation
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Gowert, N.S.; Donner, L.; Chatterjee, M.; Eisele, Y.S.; Towhid, S.T.; Munzer, P.; Walker, B.; Ogorek, I.; Borst, O.; Grandoch, M.; et al. Blood platelets in the progression of Alzheimer’s disease. PLoS ONE 2014, 9, e90523. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Goubran, H.A.; Chou, M.L.; Devos, D.; Radosevic, M. Platelet microparticles: Detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev. 2014, 28, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Thushara, R.M.; Hemshekhar, M.; Basappa; Kemparaju, K.; Rangappa, K.S.; Girish, K.S. Biologicals, platelet apoptosis and human diseases: An outlook. Crit. Rev. Oncol. Hematol. 2015, 93, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, H.; Wang, K.; Yang, J.; Yang, L.; Liu, J.; Zhang, M.; Dun, W. Correlation Between Thalamus-Related Functional Connectivity and Serum BDNF Levels During the Periovulatory Phase of Primary Dysmenorrhea. Front. Hum. Neurosci. 2019, 13, 333. [Google Scholar] [CrossRef]
- Jain, K.; Tyagi, T.; Patell, K.; Xie, Y.; Kadado, A.J.; Lee, S.H.; Yarovinsky, T.; Du, J.; Hwang, J.; Martin, K.A.; et al. Age associated non-linear regulation of redox homeostasis in the anucleate platelet: Implications for CVD risk patients. EBioMedicine 2019, 44, 28–40. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights from Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Ijsselmuiden, A.J.; Musters, R.J.; de Ruiter, G.; van Heerebeek, L.; Alderse-Baas, F.; van Schilfgaarde, M.; Leyte, A.; Tangelder, G.J.; Laarman, G.J.; Paulus, W.J. Circulating white blood cells and platelets amplify oxidative stress in heart failure. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 811–820. [Google Scholar] [CrossRef]
- Lopez, J.J.; Salido, G.M.; Gomez-Arteta, E.; Rosado, J.A.; Pariente, J.A. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J. Thromb. Haemost. 2007, 5, 1283–1291. [Google Scholar] [CrossRef]
- Leytin, V. Apoptosis in the anucleate platelet. Blood Rev. 2012, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Pietraforte, D.; Vona, R.; Marchesi, A.; de Jacobis, I.T.; Villani, A.; Del Principe, D.; Straface, E. Redox control of platelet functions in physiology and pathophysiology. Antioxid. Redox Signal. 2014, 21, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Bae, O.N.; Lim, K.M.; Noh, J.Y.; Chung, S.M.; Kim, S.H.; Chung, J.H. Trivalent methylated arsenical-induced phosphatidylserine exposure and apoptosis in platelets may lead to increased thrombus formation. Toxicol. Appl. Pharmacol. 2009, 239, 144–153. [Google Scholar] [CrossRef]
- Rautou, P.E.; Vion, A.C.; Amabile, N.; Chironi, G.; Simon, A.; Tedgui, A.; Boulanger, C.M. Microparticles, vascular function, and atherothrombosis. Circ. Res. 2011, 109, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet microvesicles in health and disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Becatti, M.; Fiorillo, C.; Gori, A.M.; Marcucci, R.; Paniccia, R.; Giusti, B.; Violi, F.; Pignatelli, P.; Gensini, G.F.; Abbate, R. Platelet and leukocyte ROS production and lipoperoxidation are associated with high platelet reactivity in Non-ST elevation myocardial infarction (NSTEMI) patients on dual antiplatelet treatment. Atherosclerosis 2013, 231, 392–400. [Google Scholar] [CrossRef]
- Hernaez, A.; Lassale, C.; Castro-Barquero, S.; Ros, E.; Tresserra-Rimbau, A.; Castaner, O.; Pinto, X.; Vazquez-Ruiz, Z.; Sorli, J.V.; Salas-Salvado, J.; et al. Mediterranean Diet Maintained Platelet Count within a Healthy Range and Decreased Thrombocytopenia-Related Mortality Risk: A Randomized Controlled Trial. Nutrients 2021, 13, 559. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- Cámara, M.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Domínguez Díaz, L.; Kardinaal, A.; van Lieshout, M. Evidence of antiplatelet aggregation effects from the consumption of tomato products, according to EFSA health claim requirements. Crit. Rev. Food Sci. Nutr. 2020, 60, 1515–1522. [Google Scholar] [CrossRef]
- Olas, B. Anti-Aggregatory Potential of Selected Vegetables-Promising Dietary Components for the Prevention and Treatment of Cardiovascular Disease. Adv. Nutr. 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003, 133, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Stampfer, M.J.; Campos, H.; Sesso, H.D.; Gaziano, J.M.; Willett, W.; Ma, J. Plasma carotenoids and tocopherols and risk of myocardial infarction in a low-risk population of US male physicians. Circulation 2003, 108, 802–807. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Raederstorff, D.; Duttaroy, A.K. Fruitflow®: The first European Food Safety Authority-approved natural cardio-protective functional ingredient. Eur. J. Nutr. 2017, 56, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Dutta-Roy, A.K.; Crosbie, L.; Gordon, M.J. Effects of tomato extract on human platelet aggregation in vitro. Platelets 2001, 12, 218–227. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; van Lieshout, M.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of antiplatelet components of tomato extract on platelet function in vitro and ex vivo: A time-course cannulation study in healthy humans. Am. J. Clin. Nutr. 2006, 84, 570–579. [Google Scholar] [CrossRef]
- Fan, D.; Tian, Z.Z.; Zuo, X.; Ya, F.L.; Yang, Y. Fruitflow, a Water-soluble Tomato Concentrate, Inhibits Platelet Activation, Aggregation and Thrombosis by Regulating the Signaling Pathway of PI3K/Akt and MAPKs. J. Sun Yat-Sen Univ. (Med. Sci.) 2020, 41, 243–250. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Song, H.J.; Zhang, X.; Horgan, G.; Duttaroy, A.K. A randomised controlled trial comparing a dietary antiplatelet, the water-soluble tomato extract Fruitflow, with 75 mg aspirin in healthy subjects. Eur. J. Clin. Nutr. 2017, 71, 723–730. [Google Scholar] [CrossRef]
- Tian, Z.; Li, K.; Fan, D.; Zhao, Y.; Gao, X.; Ma, X.; Xu, L.; Shi, Y.; Ya, F.; Zou, J.; et al. Dose-dependent effects of anthocyanin supplementation on platelet function in subjects with dyslipidemia: A randomized clinical trial. EBioMedicine 2021, 70, 103533. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Ju, L.; Yu, D.; Fang, H.; Guo, Q.; Xu, X.; Li, S.; Zhao, L. Trends and food sources composition of energy, protein and fat in Chinese residents, 1992-2012. Wei Sheng Yan Jiu = J. Hyg. Res. 2018, 47, 689–704. [Google Scholar]
- Yang, Y.; Shi, Z.; Reheman, A.; Jin, J.W.; Li, C.; Wang, Y.; Andrews, M.C.; Chen, P.; Zhu, G.; Ling, W.; et al. Plant food delphinidin-3-glucoside significantly inhibits platelet activation and thrombosis: Novel protective roles against cardiovascular diseases. PLoS ONE 2012, 7, e37323. [Google Scholar] [CrossRef]
- Ya, F.L.; Xu, X.R.; Shi, Y.L.; Gallant, R.C.; Song, F.L.; Zuo, X.; Zhao, Y.M.; Tian, Z.Z.; Zhang, C.; Xu, X.P.; et al. Coenzyme Q10 Upregulates Platelet cAMP/PKA Pathway and Attenuates Integrin alpha IIb beta 3 Signaling and Thrombus Growth. Mol. Nutr. Food Res. 2019, 63, e1900662. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Tian, Z.; Zhao, Y.; Qiu, X.; Mao, Y.; Li, K.; Shi, Y.; Zhao, D.; Liang, Y.; Ji, Q.; et al. Coenzyme Q10 supplementation improves cholesterol efflux capacity and antiinflammatory properties of high-density lipoprotein in Chinese adults with dyslipidemia. Nutrition 2022, 101, 111703. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Lopez, J.J.; Salido, G.M.; Pariente, J.A.; Rosado, J.A. Thrombin induces activation and translocation of Bid, Bax and Bak to the mitochondria in human platelets. J. Thromb. Haemost. 2008, 6, 1780–1788. [Google Scholar] [CrossRef]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Sesso, H.D. Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef]
- Leytin, V.; Allen, D.J.; Mutlu, A.; Gyulkhandanyan, A.V.; Mykhaylov, S.; Freedman, J. Mitochondrial control of platelet apoptosis: Effect of cyclosporin A, an inhibitor of the mitochondrial permeability transition pore. Lab. Investig. 2009, 89, 374–384. [Google Scholar] [CrossRef]
- Pang, A.; Cui, Y.; Chen, Y.; Cheng, N.; Delaney, M.K.; Gu, M.; Stojanovic-Terpo, A.; Zhu, C.; Du, X. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood 2018, 132, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gyulkhandanyan, A.V.; Mutlu, A.; Freedman, J.; Leytin, V. Markers of platelet apoptosis: Methodology and applications. J. Thromb. Thrombolysis 2012, 33, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Sener, A.; Ozsavci, D.; Oba, R.; Demirel, G.Y.; Uras, F.; Yardimci, K.T. Do platelet apoptosis, activation, aggregation, lipid peroxidation and platelet-leukocyte aggregate formation occur simultaneously in hyperlipidemia? Clin. Biochem. 2005, 38, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-Y.; Shi, Y.-L.; Ma, X.-L.; Tian, Z.-Z.; Zou, J.-C.; Wang, R.-J.; Mao, Y.-H.; Yang, Y. Fruitflow, a Water-soluble Tomato Extract, Regulates Platelet Oxidative Damage via Autophagy in Vitro. J. Sun Yat-Sen Univ. (Med. Sci.) 2021, 42, 321–327. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, X.Y.; Ouyang, Q.; Jiang, L.X.; Wu, Y.Q.; Jiang, Y.F. Effect of Quercetin on Apoptosis of Platelets and Its Mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 1612–1616. [Google Scholar] [CrossRef]
- Ma, J.Q.; Liu, C.M.; Yang, W. Protective effect of rutin against carbon tetrachloride-induced oxidative stress, inflammation and apoptosis in mouse kidney associated with the ceramide, MAPKs, p53 and calpain activities. Chem. Biol. Interact. 2018, 286, 26–33. [Google Scholar] [CrossRef]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef]
- Lee, S.H.; Du, J.; Stitham, J.; Atteya, G.; Lee, S.; Xiang, Y.; Wang, D.; Jin, Y.; Leslie, K.L.; Spollett, G.; et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol. Med. 2016, 8, 779–795. [Google Scholar] [CrossRef]
- Tang, W.H.; Stitham, J.; Jin, Y.; Liu, R.; Lee, S.H.; Du, J.; Atteya, G.; Gleim, S.; Spollett, G.; Martin, K.; et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 2014, 129, 1598–1609. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Zaidun, N.H.; Thent, Z.C.; Latiff, A.A. Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Sci. 2018, 208, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Whelan, S.; Luther, V.; Horgan, G.; Broom, J.I.; Webb, D.J.; Duttaroy, A.K. Effects of tomato extract on platelet function: A double-blinded crossover study in healthy humans. Am. J. Clin. Nutr. 2006, 84, 561–569. [Google Scholar] [CrossRef]
- Mone, P.; Trimarco, B.; Santulli, G. Aspirin, NOACs, warfarin: Which is the best choice to tackle cognitive decline in elderly patients? Insights from the GIRAF and ASCEND-Dementia trials presented at the AHA 2021. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, E7–E8. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Fan, D.; Li, K.; Zhao, D.; Liang, Y.; Ji, Q.; Gao, X.; Ma, X.; Zhao, Y.; Mao, Y.; et al. Four-Week Supplementation of Water-Soluble Tomato Extract Attenuates Platelet Function in Chinese Healthy Middle-Aged and Older Individuals: A Randomized, Double-Blinded, and Crossover Clinical Trial. Front. Nutr. 2022, 9, 891241. [Google Scholar] [CrossRef]
- Uddin, M.; Biswas, D.; Ghosh, A.; O’Kennedy, N.; Duttaroy, A.K. Consumption of Fruitflow® lowers blood pressure in pre-hypertensive males: A randomised, placebo controlled, double blind, cross-over study. Int. J. Food Sci. Nutr. 2018, 69, 494–502. [Google Scholar] [CrossRef]






| N = 52 | |
|---|---|
| Age, (years) | 56.13 ± 1.01 a |
| Gender (male/female) | 16/36 |
| Education attainment | |
| Primary school | 2(3.85%) |
| Middle school | 23(44.23%) |
| College | 27(51.92%) |
| Occupations | |
| Sales/workers/farmers | 18(34.61%) |
| Professionals/technicians | 29(55.76%) |
| Others | 5(9.62%) |
| Anthropometrics | |
| Weight (kg) | 64.17 ± 1.70 |
| BMI (kg/m2) | 24.57 ± 0.46 |
| NC (cm) | 34.40 ± 1.08 |
| WC (cm) | 84.99 ± 1.59 |
| WHR | 0.88 ± 0.01 |
| SBP (mmHg) | 118.32 ± 2.19 |
| DBP (mmHg) | 77.19 ± 1.47 |
| Lifestyle factors | |
| Current smoking | 2(3.8%) |
| Regular alcohol drinking | 11(21%) |
| Placebo (n = 52) | 150mg WSTC (n = 52) | |||
|---|---|---|---|---|
| Baseline | 4 Weeks | Baseline | 4 Weeks | |
| Liver function | ||||
| ALT (U/L) | 21.08 ± 1.57 a | 21.27 ± 2.03 | 21.75 ± 1.97 | 20.55 ± 1.51 |
| Total protein (g/L) | 74.19 ± 0.50 | 73.37 ± 0.45 | 73.58 ± 0.56 | 73.16 ± 0.53 |
| Albumin (g/L) | 47.05 ± 0.32 | 45.97 ± 0.33 | 46.63 ± 0.36 | 45.55 ± 0.35 |
| Albumin/Globulin | 1.77 ± 0.04 | 1.69 ± 0.03 | 1.76 ± 0.03 | 1.68 ± 0.03 |
| Renal function | ||||
| Urea (mmol/L) | 4.86 ± 1.42 | 4.97 ± 0.15 | 5.01 ± 0.17 | 4.82 ± 0.14 |
| Creatinine (μmol/L) | 78.35 ± 2.44 | 78.29 ± 2.30 | 79.21 ± 2.36 | 78.44 ± 2.38 |
| Plasma clotting times | ||||
| Prothrombin time (s) | 10.81 ± 0.73 | 11.26 ± 0.81 | 10.90 ± 0.71 | 11.16 ± 0.73 |
| APTT (s) | 28.75 ± 0.24 | 28.64 ± 0.28 | 28.68 ± 0.28 | 28.48 ± 0.25 |
| Thrombin time (s) | 18.76 ± 0.09 | 18.76 ± 0.13 | 18.77 ± 0.10 | 18.91 ± 0.11 |
| Fibrinogen (g/L) | 2.98 ± 0.06 | 3.03 ± 0.08 | 3.01 ± 0.07 | 2.92 ± 0.08 |
| Platelet parameters | ||||
| PLT (109/L) | 244.20 ± 9.05 | 253.53 ± 9.90 | 244.12 ± 8.71 | 250.69 ± 9.27 |
| MPV (fl) | 10.20 ± 0.15 | 10.18 ± 0.13 | 10.17 ± 0.15 | 10.22 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Li, K.; Fan, D.; Gao, X.; Ma, X.; Zhao, Y.; Zhao, D.; Liang, Y.; Ji, Q.; Chen, Y.; et al. Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial. Nutrients 2022, 14, 3374. https://doi.org/10.3390/nu14163374
Tian Z, Li K, Fan D, Gao X, Ma X, Zhao Y, Zhao D, Liang Y, Ji Q, Chen Y, et al. Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial. Nutrients. 2022; 14(16):3374. https://doi.org/10.3390/nu14163374
Chicago/Turabian StyleTian, Zezhong, Kongyao Li, Die Fan, Xiaoli Gao, Xilin Ma, Yimin Zhao, Dan Zhao, Ying Liang, Qiuhua Ji, Yiting Chen, and et al. 2022. "Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial" Nutrients 14, no. 16: 3374. https://doi.org/10.3390/nu14163374
APA StyleTian, Z., Li, K., Fan, D., Gao, X., Ma, X., Zhao, Y., Zhao, D., Liang, Y., Ji, Q., Chen, Y., & Yang, Y. (2022). Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial. Nutrients, 14(16), 3374. https://doi.org/10.3390/nu14163374