Genetic Predisposition, Fruit Intake and Incident Stroke: A Prospective Chinese Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Fruit Intake and Covariate Assessment
2.3. Ascertainment of Incident Stroke Events
2.4. Polygenetic Risk Score for Stroke
2.5. Statistical Analysis
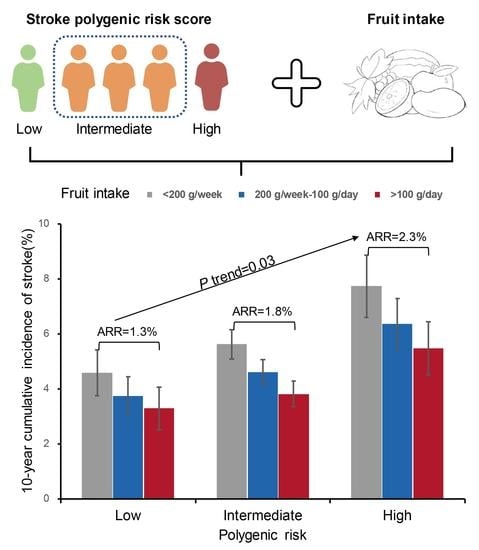
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke 2014, 45, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.Y.; He, Y.N. China National Nutrition and Health Surveillance Report: 2010–2013 Food and Nutrient Intake, 1st ed.; People’s Medical Publishing House: Beijing, China, 2018; pp. 18–24. (In Chinese) [Google Scholar]
- He, Y.; Li, Y.; Yang, X.; Hemler, E.C.; Fang, Y.; Zhao, L.; Zhang, J.; Yang, Z.; Wang, Z.; He, L.; et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: A cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019, 7, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Pulit, S.L.; Rosand, J. Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 2019, 18, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Dominiczak, A.F. Genomics of hypertension: The road to precision medicine. Nat. Rev. Cardiol. 2020, 18, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M. Biomarkers for type 2 diabetes. Mol. Metab. 2019, 27S, S139–S146. [Google Scholar] [CrossRef]
- Lu, X.; Peloso, G.M.; Liu, D.J.; Wu, Y.; Zhang, H.; Zhou, W.; Li, J.; Tang, C.S.; Dorajoo, R.; Li, H.; et al. Exome chip meta-analysis identifies novel loci and East Asian-specific coding variants that contribute to lipid levels and coronary artery disease. Nat. Genet 2017, 49, 1722–1730. [Google Scholar] [CrossRef]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Bluher, M.; Kovacs, P.; Bottcher, Y. Genetics and epigenetics in obesity. Metabolism 2019, 92, 37–50. [Google Scholar] [CrossRef]
- Rutten-Jacobs, L.C.; Larsson, S.C.; Malik, R.; Rannikmae, K.; Sudlow, C.L.; Dichgans, M.; Markus, H.S.; Traylor, M. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: Cohort study of 306 473 UK Biobank participants. BMJ 2018, 363, k4168. [Google Scholar] [CrossRef] [Green Version]
- Abraham, G.; Malik, R.; Yonova-Doing, E.; Salim, A.; Wang, T.; Danesh, J.; Butterworth, A.S.; Howson, J.M.M.; Inouye, M.; Dichgans, M. Genomic risk score offers predictive performance comparable to clinical risk factors for ischaemic stroke. Nat. Commun. 2019, 10, 5819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, G.; Rutten-Jacobs, L.; Inouye, M. Risk Prediction Using Polygenic Risk Scores for Prevention of Stroke and Other Cardiovascular Diseases. Stroke 2021, 52, 2983–2991. [Google Scholar] [CrossRef]
- Wang, T.; Heianza, Y.; Sun, D.; Zheng, Y.; Huang, T.; Ma, W.; Rimm, E.B.; Manson, J.E.; Hu, F.B.; Willett, W.C.; et al. Improving fruit and vegetable intake attenuates the genetic association with long-term weight gain. Am. J. Clin. Nutr. 2019, 110, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Z.; Cui, Q.; Liu, F.; Li, J.; Niu, X.; Shen, C.; Hu, D.; Huang, K.; Chen, J.; et al. A polygenic risk score improves risk stratification of coronary artery disease: A large-scale prospective Chinese cohort study. Eur. Heart J. 2022, 43, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Hu, D.; Chen, J.; Li, Y.; Huang, J.; Liu, X.; Liu, F.; Cao, J.; Shen, C.; et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: The China-PAR project (Prediction for ASCVD Risk in China). Circulation 2016, 134, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Yusuf, S.; Chow, C.K.; Dehghan, M.; Corsi, D.J.; Lock, K.; Popkin, B.; Rangarajan, S.; Khatib, R.; Lear, S.A.; et al. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Glob. Health 2016, 4, e695–e703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Niu, X.; Shen, C.; Liu, F.; Liu, Z.; Huang, K.; Wang, L.; Li, J.; Hu, D.; Zhao, Y.; et al. Development and Validation of a Polygenic Risk Score for Stroke in the Chinese Population. Neurology 2021, 97, e619–e628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Huang, Z.-P.; Zhang, X.; He, L.; Willett, W.; Wang, J.-L.; Hasegawa, K.; Chen, J.-S. Reproducibility and Validity of a Chinese Food Frequency Questionnaire. Biomed. Environ. Sci. 2010, 23, 1–38. [Google Scholar] [CrossRef]
- Han, C.; Liu, F.; Yang, X.; Chen, J.; Li, J.; Cao, J.; Li, Y.; Shen, C.; Yu, L.; Liu, Z.; et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: The China-PAR project. Sci. China Life Sci. 2018, 61, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Li, J.; Yang, X.; Chen, J.; Cao, J.; Wu, X.; Lu, X.; Huang, J.; Li, Y.; et al. Tea consumption and the risk of atherosclerotic cardiovascular disease and all-cause mortality: The China-PAR project. Eur. J. Prev. Cardiol. 2020, 27, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. Poisson regression adjustment of event rates and its macro procedure ADJ_POIS. In Proceedings of the SAS Conference Proceedings: SAS Users Group International 24, Miami Beach, FL, USA, 11–14 April 1999. [Google Scholar]
- Knol, M.J.; VanderWeele, T.J.; Groenwold, R.H.; Klungel, O.H.; Rovers, M.M.; Grobbee, D.E. Estimating measures of interaction on an additive scale for preventive exposures. Eur. J. Epidemiol. 2011, 26, 433–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Yang, X.; Liang, F.; Huang, K.; Liu, F.; Li, J.; Xiao, Q.; Chen, J.; Liu, X.; Cao, J.; et al. Benefits of active commuting on cardiovascular health modified by ambient fine particulate matter in China: A prospective cohort study. Ecotoxicol. Environ. Saf. 2021, 224, 112641. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.; Cole, S.R.; Gange, S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009, 170, 244–256. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; Katzke, V.; Kuhn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh fruit consumption and major cardiovascular disease in China. N. Engl. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Oude Griep, L.M.; Verschuren, W.M.; Kromhout, D.; Ocke, M.C.; Geleijnse, J.M. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke 2011, 42, 3190–3195. [Google Scholar] [CrossRef] [Green Version]
- Do, R.; Xie, C.; Zhang, X.; Mannisto, S.; Harald, K.; Islam, S.; Bailey, S.D.; Rangarajan, S.; McQueen, M.J.; Diaz, R.; et al. The effect of chromosome 9p21 variants on cardiovascular disease may be modified by dietary intake: Evidence from a case/control and a prospective study. PLoS Med. 2011, 8, e1001106. [Google Scholar] [CrossRef] [PubMed]
- Hindy, G.; Ericson, U.; Hamrefors, V.; Drake, I.; Wirfalt, E.; Melander, O.; Orho-Melander, M. The chromosome 9p21 variant interacts with vegetable and wine intake to influence the risk of cardiovascular disease: A population based cohort study. BMC Med. Genet 2014, 15, 1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murgia, C.; Adamski, M.M. Translation of Nutritional Genomics into Nutrition Practice: The Next Step. Nutrients 2017, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Mullins, V.A.; Bresette, W.; Johnstone, L.; Hallmark, B.; Chilton, F.H. Genomics in Personalized Nutrition: Can You “Eat for Your Genes”? Nutrients 2020, 12, 3118. [Google Scholar] [CrossRef] [PubMed]
- Said, M.A.; Verweij, N.; van der Harst, P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.Y.; Li, H.B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | No Incident Stroke | Incident Stroke | p Value |
|---|---|---|---|
| (n = 32,285) | (n = 2586) | ||
| Age, year | 55 ± 10 | 60 ± 9 | <0.001 |
| Female, n (%) | 18,701 (58) | 1427 (55) | 0.007 |
| Northern, n (%) | 15,389 (48) | 1437 (56) | <0.001 |
| Urban residence, n (%) | 5462 (17) | 151 (5.8) | <0.001 |
| High-school or above, n (%) | 15,544 (48) | 779 (30) | <0.001 |
| Current smoker, n (%) | 7560 (23) | 633 (25) | 0.22 |
| Alcohol drinker, n (%) | 7571 (23) | 521 (20) | <0.001 |
| Family history of stroke, n (%) | 3188 (10) | 303 (12) | 0.003 |
| Ideal physical activity, n (%) | 20,244 (63) | 1662 (64) | 0.11 |
| Ideal diet score, n (%) | 17,019 (53) | 1086 (42) | <0.001 |
| Body mass index, kg/m2 | 24 ± 4 | 24 ± 4 | <0.001 |
| Waist circumference, cm | 82 ± 10 | 84 ± 10 | <0.001 |
| Hypertension, n (%) | 11,945 (37) | 1435 (56) | <0.001 |
| Diabetes mellitus, n (%) | 2596 (8.3) | 329 (13) | <0.001 |
| Dyslipidemia, n (%) | 10,047 (32) | 1025 (41) | <0.001 |
| Fruit intake, g/day | 85 ± 86 | 66 ± 78 | <0.001 |
| Vegetable intake, g/day | 335 ± 157 | 327 ± 158 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, J.; Liu, F.; Huang, K.; Yang, X.; Liu, X.; Cao, J.; Chen, S.; Shen, C.; Yu, L.; et al. Genetic Predisposition, Fruit Intake and Incident Stroke: A Prospective Chinese Cohort Study. Nutrients 2022, 14, 5056. https://doi.org/10.3390/nu14235056
Wang J, Li J, Liu F, Huang K, Yang X, Liu X, Cao J, Chen S, Shen C, Yu L, et al. Genetic Predisposition, Fruit Intake and Incident Stroke: A Prospective Chinese Cohort Study. Nutrients. 2022; 14(23):5056. https://doi.org/10.3390/nu14235056
Chicago/Turabian StyleWang, Jun, Jianxin Li, Fangchao Liu, Keyong Huang, Xueli Yang, Xiaoqing Liu, Jie Cao, Shufeng Chen, Chong Shen, Ling Yu, and et al. 2022. "Genetic Predisposition, Fruit Intake and Incident Stroke: A Prospective Chinese Cohort Study" Nutrients 14, no. 23: 5056. https://doi.org/10.3390/nu14235056
APA StyleWang, J., Li, J., Liu, F., Huang, K., Yang, X., Liu, X., Cao, J., Chen, S., Shen, C., Yu, L., Lu, F., Zhao, L., Li, Y., Hu, D., Huang, J., Gu, D., & Lu, X. (2022). Genetic Predisposition, Fruit Intake and Incident Stroke: A Prospective Chinese Cohort Study. Nutrients, 14(23), 5056. https://doi.org/10.3390/nu14235056