Meal Timing and Sleeping Energy Metabolism
Abstract
:1. Introduction
2. Effect of Meal Pattern on Sleeping Energy Metabolism
3. The Relationship between Energy Metabolism and Sleep
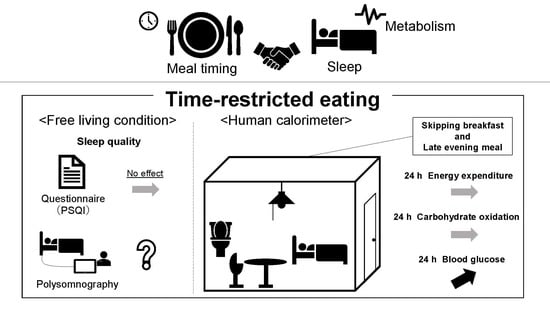
4. Effects of Time-Restricted Eating on Sleep, and Its Mechanisms, Benefits and Translational Potential
5. Implications and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolaidis, S. Metabolic mechanism of wakefulness (and hunger) and sleep (and satiety): Role of adenosine triphosphate and hypocretin and other peptides. Metabolism 2006, 55 (Suppl. 2), S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Wroblewski, K.; Kahn, E.; Kilkus, J.; Schoeller, D.A. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults with Overweight in Real-life Settings: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Michael, P. COOKED A Natural History of Transformation; Penguin Books: Baltimore, MD, USA, 2014; p. 9. [Google Scholar]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A. Nonexercise activity thermogenesis (NEAT): Environment and biology. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E675–E685, published correction appears in Am. J. Physiol. Endocrinol. Metab. 2005, 288, E285. [Google Scholar] [CrossRef] [PubMed]
- Fontvieille, A.M.; Rising, R.; Spraul, M.; Larson, D.E.; Ravussin, E. Relationship between sleep stages and metabolic rate in humans. Am. J. Physiol. 1994, 267 Pt 1, E732–E737. [Google Scholar] [CrossRef]
- Sato, M.; Nakamura, K.; Ogata, H.; Miyashita, A.; Nagasaka, S.; Omi, N.; Yamaguchi, S.; Hibi, M.; Umeda, T.; Nakaji, S.; et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011, 5, e169–e266. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pract. 2014, 8, e201–e298. [Google Scholar] [CrossRef]
- Ogata, H.; Kayaba, M.; Tanaka, Y.; Yajima, K.; Iwayama, K.; Ando, A.; Park, I.; Kiyono, K.; Omi, N.; Satoh, M.; et al. Effect of skipping breakfast for 6 days on energy metabolism and diurnal rhythm of blood glucose in young healthy Japanese males. Am. J. Clin. Nutr. 2019, 110, 41–52. [Google Scholar] [CrossRef]
- Nas, A.; Mirza, N.; Hägele, F.; Kahlhöfer, J.; Keller, J.; Rising, R.; Kufer, T.A.; Bosy-Westphal, A. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am. J. Clin. Nutr. 2017, 105, 1351–1361. [Google Scholar] [CrossRef]
- Reichmuth, K.J.; Austin, D.; Skatrud, J.B.; Young, T. Association of sleep apnea and type II diabetes: A population-based study. Am. J. Respir. Crit. Care Med. 2005, 172, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.R.; Herdegen, J.; Fogelfeld, L.; Shott, S.; Mazzone, T. Type 2 Diabetes, Glycemic Control, and Continuous Positive Airway Pressure in Obstructive Sleep Apnea. Arch. Intern. Med. 2005, 165, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M. Diabetes: Advances in Diagnosis and Treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Terauchi, Y. Continuous glucose monitoring. Mod. Media 2021, 67, 12–17. [Google Scholar]
- Ogata, H.; Tokuyama, K.; Nagasaka, S.; Ando, A.; Kusaka, I.; Sato, N.; Goto, A.; Ishibashi, S.; Kiyono, K.; Struzik, Z.; et al. Long-range negative correlation of glucose dynamics in humans and its breakdown in diabetes mellitus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1638–R1643. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.L.; Philp, A.; Harrison, M.; Bone, A.J.; Watt, P.W. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med. Sci. Sports Exerc. 2006, 38, 201–207. [Google Scholar] [CrossRef]
- Hasson, R.E.; Freedson, P.S.; Braun, B. Use of continuous glucose monitoring in normoglycemic, insulin-resistant women. Eur. J. Appl. Physiol. 2010, 108, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Hoss, U.; Budiman, E.S. Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology. Diabetes Technol. Ther. 2017, 19, S44–S50. [Google Scholar] [CrossRef] [PubMed]
- Abbott Japan Co., Ltd. FreeStyle Libre Pro Performance Sheet; Abbott Japan Co., Ltd.: Tokyo, Japan, 2016. [Google Scholar]
- Howard, R.; Guo, J.; Hall, K.D. Imprecision nutrition? Different simultaneous continuous glucose monitors provide discordant meal rankings for incremental postprandial glucose in subjects without diabetes. Am. J. Clin. Nutr. 2020, 112, 1114–1119. [Google Scholar] [CrossRef]
- Taheri, S. The link between short sleep duration and obesity: We should recommend more sleep to prevent obesity. Arch. Dis. Child. 2006, 91, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Chambers, E.S.; Bridge, M.W.; Jones, D.A. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. 2009, 587 Pt 8, 1779–1794. [Google Scholar] [CrossRef]
- Landström, U.; Knutsson, A.; Lennernäs, M. Field studies on the effects of food content on wakefulness. Nutr. Health 2000, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Baulk, S.D. Awareness of sleepiness when driving. Psychophysiology 2004, 41, 161–165. [Google Scholar] [CrossRef]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430, published correction appears in Am. J. Clin. Nutr. 2007, 86, 809. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Horne, J.A. A high sugar content, low caffeine drink does not alleviate sleepiness but may worsen it. Hum. Psychopharmacol. 2006, 21, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Varin, C.; Rancillac, A.; Geoffroy, H.; Arthaud, S.; Fort, P.; Gallopin, T. Glucose Induces Slow-Wave Sleep by Exciting the Sleep-Promoting Neurons in the Ventrolateral Preoptic Nucleus: A New Link between Sleep and Metabolism. J. Neurosci. 2015, 35, 9900–9911. [Google Scholar] [CrossRef] [PubMed]
- Danguir, J.; Nicolaidis, S. Chronic intracerebroventricular infusion of insulin causes selective increase of slow wave sleep in rats. Brain Res. 1984, 306, 97–103. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.-W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Wurtman, J.J.; Regan, M.M.; McDermott, J.M.; Tsay, R.H.; Breu, J.J. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am. J. Clin. Nutr. 2003, 77, 128–132. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Pavlou, V.; Lin, S.; Wiseman, E.; A Varady, K. The effect of 4-h versus 6-h time restricted feeding on sleep quality, duration, insomnia severity and obstructive sleep apnea in adults with obesity. Nutr. Health 2022, 28, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Burgess, H.J.; Varady, K.A. Effect of 8-h time-restricted feeding on sleep quality and duration in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 903–906. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, B.J.; Jung, A.R.; Kim, J.; Ju, H.J.; Kim, Y.I. The Impact of Time-Restricted Diet on Sleep and Metabolism in Obese Volunteers. Medicina 2020, 56, 540. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Zavecz, Z.; Nagy, T.; Galkó, A.; Nemeth, D.; Janacsek, K. The relationship between subjective sleep quality and cognitive performance in healthy young adults: Evidence from three empirical studies. Sci. Rep. 2020, 10, 4855. [Google Scholar] [CrossRef]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef]
- Seol, J.; Lee, J.; Park, I.; Tokuyama, K.; Fukusumi, S.; Kokubo, T.; Yanagisawa, M.; Okura, T. Bidirectional associations between physical activity and sleep in older adults: A multilevel analysis using polysomnography. Sci. Rep. 2022, 12, 15399. [Google Scholar] [CrossRef]
- Isa, S.; Sasaki, S. Dietary Reference Intakes for Japanese (2020); Daiichisyuppan: Tokyo, Japan, 2020; p. 24. [Google Scholar]
- Goris, A.H.; Westerterp, K.R. Underreporting of habitual food intake is explained by undereating in highly motivated lean women. J. Nutr. 1999, 129, 878–882. [Google Scholar] [CrossRef]
- Archundia Herrera, M.C.; Chan, C.B. Narrative Review of New Methods for Assessing Food and Energy Intake. Nutrients 2018, 10, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, R.; Sun, Y.; Jiang, Y.; Ye, L.; Hong, J.; Wang, W. Effects of Time-Restricted Feeding on Energy Balance: A Cross-Over Trial in Healthy Subjects. Front. Endocrinol. 2022, 13, 870054. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Smith, J.; Kasriel, S.; Barlow, J.; Lynn, M.J.; Nixon, D.; Lawson, D.H. Energy malabsorption: Measurement and nutritional consequences. Am. J. Clin. Nutr. 1981, 34, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Basolo, A.; Hohenadel, M.; Ang, Q.Y.; Piaggi, P.; Heinitz, S.; Walter, M.; Walter, P.; Parrington, S.; Trinidad, D.D.; von Schwartzenberg, R.J.; et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat. Med. 2020, 26, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hepler, C.; Weidemann, B.J.; Waldeck, N.J.; Marcheva, B.; Cedernaes, J.; Thorne, A.K.; Kobayashi, Y.; Nozawa, R.; Newman, M.V.; Gao, P.; et al. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 2022, 378, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Notley, S.R.; Gagnon, D. Direct calorimetry: A brief historical review of its use in the study of human metabolism and thermoregulation. Eur. J. Appl. Physiol. 2017, 117, 1765–1785. [Google Scholar] [CrossRef]
- Chen, K.Y.; Smith, S.; Ravussin, E.; Krakoff, J.; Plasqui, G.; Tanaka, S.; Murgatroyd, P.; Brychta, R.; Bock, C.; Carnero, E.; et al. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0): A Guide to Conducting and Reporting Human Whole-Room Calorimeter Studies. Obesity 2020, 28, 1613–1625. [Google Scholar] [CrossRef]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Ishihara, A.; Park, I.; Suzuki, Y.; Yajima, K.; Cui, H.; Yanagisawa, M.; Sano, T.; Kido, J.; Tokuyama, K. Metabolic responses to polychromatic LED and OLED light at night. Sci. Rep. 2021, 11, 12402. [Google Scholar] [CrossRef]
- Tokuyama, K.; Ogata, H.; Katayose, Y.; Satoh, M. Algorithm for transient response of whole body indirect calorimeter: Deconvolution with a regularization parameter. J. Appl. Physiol. (1985) 2009, 106, 640–650. [Google Scholar] [CrossRef]
- Peters, B.; Koppold-Liebscher, D.A.; Schuppelius, B.; Steckhan, N.; Pfeiffer, A.F.H.; Kramer, A.; Michalsen, A.; Pivovarova-Ramich, O. Effects of Early vs. Late Time-Restricted Eating on Cardiometabolic Health, Inflammation, and Sleep in Overweight and Obese Women: A Study Protocol for the ChronoFast Trial. Front. Nutr. 2021, 8, 765543. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Dashti, H.S.; Madrid-Valero, J.J.; Madrid, J.A.; Saxena, R.; Scheer, F.A.; Ordoñana, J.R.; Garaulet, M. Heritability of the timing of food intake. Clin. Nutr. 2019, 38, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Garaulet, M.; Scheer, F.A.J.L. Meal timing and obesity: Interactions with macronutrient intake and chronotype. Int. J. Obes. 2019, 43, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of night eating habits with metabolic syndrome and its components: A longitudinal study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef] [PubMed]
- Bin Yan, B.; Fan, Y.; Zhao, B.; He, X.; Yang, J.; Chen, C.; Ma, X. Association Between Late Bedtime and Diabetes Mellitus: A Large Community-Based Study. J. Clin. Sleep Med. 2019, 15, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Brereton, N.; Schweitzer, A.; Cotter, M.; Duan, D.; Børsheim, E.; Wolfe, R.R.; Pham, L.V.; Polotsky, V.Y.; Jun, J.C. Metabolic Effects of Late Dinner in Healthy Volunteers-A Randomized Crossover Clinical Trial. J. Clin. Endocrinol. Metab. 2020, 105, 2789–2802. [Google Scholar] [CrossRef]
- Ramsay, S.A.; Bloch, T.D.; Marriage, B.; Shriver, L.H.; Spees, C.K.; Taylor, C.A. Skipping breakfast is associated with lower diet quality in young US children. Eur. J. Clin. Nutr. 2018, 72, 548–556. [Google Scholar] [CrossRef]
- Yoshimura, E.; Hatamoto, Y.; Yonekura, S.; Tanaka, H. Skipping breakfast reduces energy intake and physical activity in healthy women who are habitual breakfast eaters: A randomized crossover trial. Physiol. Behav. 2017, 174, 89–94. [Google Scholar] [CrossRef]
- Silber, B.Y.; Schmitt, J.A. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci. Biobehav. Rev. 2010, 34, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Etherton, G.M.; Kochar, M.S. Coffee Facts and controversies. Arch. Fam. Med. 1993, 2, 317–322. [Google Scholar] [CrossRef]
- Landolt, H.P.; Dijk, D.J.; Gaus, S.E.; Borbély, A.A. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology 1995, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, L.J.; Jarvik, M.E. Comparative stimulant and diuretic actions of caffeine and theobromine in man. Clin. Pharmacol. Ther. 1970, 11, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G. Ephedrine, xanthines and prostaglandin-inhibitors: Actions and interactions in the stimulation of thermogenesis. Int. J. Obes. Relat. Metab. Disord. 1993, 17 (Suppl. 1), S35–S40. [Google Scholar] [PubMed]
- Racotta, I.S.; Leblanc, J.; Richard, D. The effect of caffeine on food intake in rats: Involvement of corticotropin-releasing factor and the sympatho-adrenal system. Pharmacol. Biochem. Behav. 1994, 48, 887–892. [Google Scholar] [CrossRef]
- Tremblay, A.; Masson, E.; Leduc, S.; Houde, A.; Després, J.-P. Caffeine reduces spontaneous energy intake in men but not in women. Nutr. Res. 1988, 8, 553–558. [Google Scholar] [CrossRef]
- Munsters, M.J.; Saris, W.H. Effects of meal frequency on metabolic profiles and substrate partitioning in lean healthy males. PLoS ONE 2012, 7, e38632. [Google Scholar] [CrossRef]
- Ohkawara, K.; Cornier, M.A.; Kohrt, W.M.; Melanson, E.L. Effects of increased meal frequency on fat oxidation and perceived hunger. Obesity 2013, 21, 336–343. [Google Scholar] [CrossRef]
- Taylor, M.A.; Garrow, J.S. Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 519–528. [Google Scholar] [CrossRef]
- Dallosso, H.M.; Murgatroyd, P.R.; James, W.P. Feeding frequency and energy balance in adult males. Hum. Nutr. Clin. Nutr. 1982, 36, 25–39. [Google Scholar]
- Verboeket-van de Venne, W.P.; Westerterp, K.R. Influence of the feeding frequency on nutrient utilization in man: Consequences for energy metabolism. Eur. J. Clin. Nutr. 1991, 45, 161–169. [Google Scholar]
- Verboeket-Van De Venne, W.P.H.G.; Westerterp, K.R.; Kester, A.D.M. Effect of the pattern of food intake on human energy metabolism. Br. J. Nutr. 1993, 70, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Timlin, M.T.; Pereira, M.A. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr. Rev. 2007, 65 Pt 1, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh-Taskar, P.R.; Nicklas, T.A.; O’Neil, C.E.; Keast, D.R.; Radcliffe, J.D.; Cho, S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: The National Health and Nutrition Examination Survey 1999–2006. J. Am. Diet. Assoc. 2010, 110, 869–878. [Google Scholar] [CrossRef]
- Kerver, J.M.; Yang, E.J.; Obayashi, S.; Bianchi, L.; Song, W.O. Meal and snack patterns are associated with dietary intake of energy and nutrients in US adults. J. Am. Diet. Assoc. 2006, 106, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Myers, L.; Reger, C.; Beech, B.; Berenson, G.S. Impact of breakfast consumption on nutritional adequacy of the diets of young adults in Bogalusa, Louisiana: Ethnic and gender contrasts. J. Am. Diet. Assoc. 1998, 98, 1432–1438. [Google Scholar] [CrossRef]
- Fukushige, H.; Fukuda, Y.; Tanaka, M.; Inami, K.; Wada, K.; Tsumura, Y.; Kondo, M.; Harada, T.; Wakamura, T.; Morita, T. Effects of tryptophan-rich breakfast and light exposure during the daytime on melatonin secretion at night. J. Physiol. Anthropol. 2014, 33, 33. [Google Scholar] [CrossRef]
- Tahara, Y.; Otsuka, M.; Fuse, Y.; Hirao, A.; Shibata, S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J. Biol. Rhythm. 2011, 26, 230–240. [Google Scholar] [CrossRef]
- Rampersaud, G.C.; Pereira, M.A.; Girard, B.L.; Adams, J.; Metzl, J.D. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J. Am. Diet. Assoc. 2005, 105, 743–762. [Google Scholar] [CrossRef]



| Duration | Eating Window | Time | Sleep | Reference |
|---|---|---|---|---|
| 16, 12 weeks | 10 h | self-selected | PSQI didn’t change | [4,32] |
| 8 weeks | 4 h | 15:00–19:00 | [33] | |
| 8 weeks | 6 h | 13:00–19:00 | [33] | |
| 12 weeks | 8 h | 10:00–18:00 | [34] | |
| 5 weeks | 9 h | 6:00–15:00 | [35] | |
| 5 weeks | 9 h | 11:00–20:00 | [35] | |
| 4 weeks | 8 h | 12:00–20:00 | [36] * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshitake, R.; Park, I.; Ogata, H.; Omi, N. Meal Timing and Sleeping Energy Metabolism. Nutrients 2023, 15, 763. https://doi.org/10.3390/nu15030763
Yoshitake R, Park I, Ogata H, Omi N. Meal Timing and Sleeping Energy Metabolism. Nutrients. 2023; 15(3):763. https://doi.org/10.3390/nu15030763
Chicago/Turabian StyleYoshitake, Rikako, Insung Park, Hitomi Ogata, and Naomi Omi. 2023. "Meal Timing and Sleeping Energy Metabolism" Nutrients 15, no. 3: 763. https://doi.org/10.3390/nu15030763
APA StyleYoshitake, R., Park, I., Ogata, H., & Omi, N. (2023). Meal Timing and Sleeping Energy Metabolism. Nutrients, 15(3), 763. https://doi.org/10.3390/nu15030763