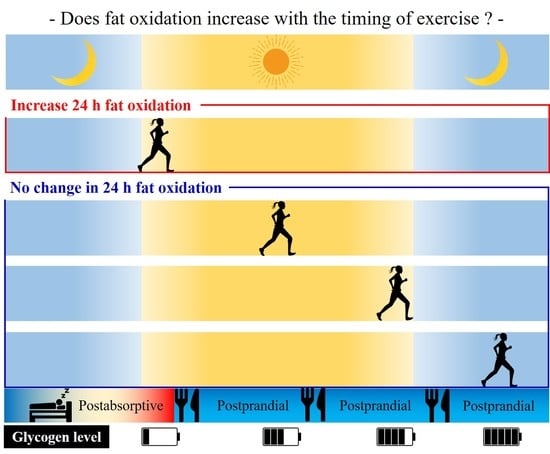
Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h
Abstract
:1. Introduction
2. Effects of Exercise Timing on Fat Oxidation during Exercise
3. Effects of Exercise Timing on 24 h Fat Oxidation: Whole-Room Metabolic Chamber Study
| Participants | Exercise | Effect of Intensity | Effect of Exercise | Ref. | |
|---|---|---|---|---|---|
| Intensity | Duration | ||||
| Lean female | 50%VO2max | 1 h | NS | [41] | |
| 100% VO2max | 2 min × 15 trials | ||||
| Obese male | 38%Wmax | 1 h | NS | [42] | |
| 80/50% Wmax | 6 sets of 2.5 min each | ||||
| Lean female and male | No exercise | NS | NS | [39] | |
| 40% VO2max | 100 min | ||||
| 70% VO2max | 60 min | ||||
| Obese female and male | No exercise | NS | NS | [43] | |
| 40% VO2max | 60 min | ||||
| 70% VO2max | 30 min | ||||
| Lean female and male | No exercise | NS | NS | ||
| 40% VO2max | 60 min | ||||
| 70% VO2max | 30 min | ||||
| Young male | No exercise | NS | [44] | ||
| 60% VO2max | 300 kcal | ||||
| Old male | No exercise | NS | |||
| 60% VO2max | 300 kcal | ||||
| Lean sedentary male | 55%VO2max | 60 min | NS | [40] | |
| Lean trained male | 55%VO2max | 60 min | |||
| Obese sedentary male | 55%VO2max | 60 min | |||
| Male | 50%VO2max | 60 min | NS | [45] | |
4. Effects of Exercise Timing on Glycogen Metabolism: 13C MRS Study
5. Limitations and Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sports and Exercise. Bureau of Labor Statistics. United States Department of Labor. Available online: http://www.bls.gov/spotlight/2008/sports/ (accessed on 18 January 2023).
- Survey on Time Use and Leisure Activities. Statistics Bureau, Ministry of Internal Affairs and Communications. Available online: https://www.e-stat.go.jp/en/stat-search/files?page=1&toukei=00200533&tstat=000001158160 (accessed on 18 January 2023).
- Heikura, I.A.; Stellingwerff, T.; Burke, L.M. Self-reported periodization of nutrition in elite female and male runners and race walkers. Front. Physiol. 2018, 9, 1732. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Rhodes, R.E.; Janssen, I.; Bredin, S.S.D.; Warburton, D.E.R.; Bauman, A. Physical activity: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 942–975. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behavior. Br. J. Sport. Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; US Dept. of Health and Human Services: Wasington, DC, USA, 2018. [Google Scholar]
- Ishay, Y.; Kolben, Y.; Kessler, A.; Ilan, Y. Role of circadian rhythm and autonomic nervous system in liver function: A hypothetical basis for improving the management of hepatic encephalopathy. Am. J. Physiol. 2021, 321, G400–G412. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Kim, H.K.; Radak, Z.; Takahashi, M.; Inami, T.; Shibata, S. Chrono-exercise: Time-of-day-dependent physiological responses to exercise. Sport. Med. Health Sci. 2022, in press. [Google Scholar] [CrossRef]
- Bachman, J.J.; Deitrick, R.W.; Hillman, A.R. Exercising in the fasted state reduced 24-hour energy intake in active male adults. J. Nutr. Metab. 2016, 2016, 1984198. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Ogata, H.; Kayaba, M.; Ando, A.; Park, I.; Yajima, K.; Araki, A.; Suzuki, C.; Osumi, H.; Zhang, S.; et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J. Appl. Physiol. 2020, 128, 847–854. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hashimoto, S.; Takasu, N.N.; Tanahashi, U.; Nishide, S.; Honma, S.; Honma, K. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am. J. Physiol. 2015, 309, R1112–R1121. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.O.; Coconcelli, L.; Kruel, L.F.M. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, J.M.; Rosenberg, R.C.; Rynders, C.A.; Melanson, E.L.; Catenacci, V.A.; Creasy, S.A. Examining the role of exercise timing in weight management: A review. Int. J. Sport. Med. 2021, 42, 967–978. [Google Scholar] [CrossRef]
- Gillen, J.B.; Percival, M.E.; Ludzki, A.; Tarnopolsky, M.A.; Gibala, M.J. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 2013, 21, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Pedersen, K.; Christensen, B.; Aagaard, P.; Brandt, N.; Kiens, B. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 2009, 197, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A.; Wilborn, C.D.; Krieger, J.W.; Sonmez, G.T. Body composition changes associated with fasted versus non-fasted aerobic exercise. J. Int. Soc. Sport. Nutr. 2014, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Souissi, N. The effect of training at a specific time of day: A review. J. Strength Cond. Res. 2012, 26, 1984–2005. [Google Scholar] [CrossRef] [PubMed]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Mancilla, R.; Krook, A.; Schrauwen, P.; Hesselink, M.K.C. Diurnal regulation of peripheral glucose metabolism: Potential effects of exercise timing. Obesity 2020, 28, S38–S45. [Google Scholar] [CrossRef]
- Terada, T.; Wilson, B.J.; Myette-Cote, E.; Kuzik, N.; Bell, G.J.; McCarggar, L.J.; Boule, N.G. Targeting specific interstitial glycemic parameters with highintensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism 2016, 65, 599–608. [Google Scholar] [CrossRef]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [Green Version]
- Amaro-Gahete, F.J.; Jurado-Fasoli, L.; Trivino, A.R.; Sanchez-Delgado, G.; De-la-O, A.; Helge, J.W.; Ruiz, J.R. Diurnal variation of maximal fat-oxidation rate in trained male athletes. Int. J. Sport Physiol. Perform. 2019, 14, 1140–1146. [Google Scholar] [CrossRef]
- Sharma, P.; Agarwal, M. Diurnal variation of fat oxidation rate and energy expenditure in an acute bout of endurance exercise by young healthy males. J. Fam. Med. Prim. Care 2022, 11, 240–244. [Google Scholar]
- Kim, H.K.; Konishi, M.; Takahashi, M.; Tabata, H.; Endo, N.; Numao, S.; Lee, S.K.; Kim, Y.H.; Suzuki, K.; Sakamoto, S. Effects of acute endurance exercise performed in the morning and evening on inflammatory cytokine and metabolic hormone responses. PLoS ONE 2015, 10, e0137567. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, S.; Shibata, S. Time-of-day-dependent physiological responses to meal and exercise. Front. Physiol. 2020, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Maughan, R.J.; Fallah, J.; Coyle, E.F. The effects of fasting on metabolism and performance. Br. J. Sport. Med. 2010, 44, 490–494. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol. 1997, 273, E768–E775. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brooks, G.A. Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men. J. Appl. Physiol. 1999, 86, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Bielinski, Y.; Schutz, Y.; Jequier, E. Energy metabolism during the postexercise recovery in man. Am. J. Clin. Nutr. 1985, 42, 69–82. [Google Scholar] [CrossRef]
- Bahr, R.; Sejersted, O.M. Effect of intensity of exercise on excess postexercise O2 consumption. Metabolism 1991, 40, 836–841. [Google Scholar] [CrossRef]
- Kuo, C.C.; Fattor, J.A.; Henderson, G.C.; Brooks, G.A. Lipid oxidation in fit young adults during postexercise recovery. J. Appl. Physiol. 2005, 99, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Børsheim, E.; Bahr, R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sport. Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, C.A.; Brooks, G.A. Metabolic basis of post-exercise oxygen consumption: A review. Med. Sci. Sport. Exerc. 1984, 16, 29–43. [Google Scholar] [CrossRef]
- Iwayama, K.; Miyashita, M.; Tokuyama, K. Changes in substrate oxidation persist overnight after a marathon race. Jpn. J. Phys. Fit. Sport. Med. 2008, 57, 163–168. [Google Scholar] [CrossRef]
- Chen, K.; Smith, S.; Ravussin, E.; Krakoff, J.; Plasqui, G.; Tanaka, S.; Murgatroyd, P.; Brychta, R.; Bock, C.; Carnero, E.; et al. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0): A guide to conducting and reporting human whole-room calorimeter studies. Obesity 2020, 28, 1613–1625. [Google Scholar] [CrossRef]
- Tokuyama, K.; Ogata, H.; Katayose, Y.; Satoh, M. Algorithm for transient response of whole body indirect calorimeter: Deconvolution with a regularization parameter. J. Appl. Physiol. 2009, 106, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Melanson, E.L.; Sharp, T.A.; Seagle, H.M.; Horton, T.J.; Donahoo, W.T.; Grunwald, G.K.; Hamilton, J.T.; Hill, J.O. Effect of exercise intensity on 24-h energy expenditure and nutrient oxidation. J. Appl. Physiol. 2002, 92, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Melanson, E.L.; Gozansky, W.S.; Barry, D.W.; Maclean, P.S.; Grunwald, G.K.; Hill, J.O. When energy balance is maintained, exercise does not induce negative fat balance in lean sedentary, obese sedentary, or lean endurance-trained individuals. J. Appl. Physiol. 2009, 107, 1847–1856. [Google Scholar] [CrossRef] [Green Version]
- Treuth, M.S.; Hunter, G.R.; Williams, M. Effects of exercise intensity on 24-h energy expenditure and substrate oxidation. Med. Sci. Sport. Exerc. 1996, 28, 1138–1143. [Google Scholar] [CrossRef]
- Saris, W.H.M.; Schrauwen, P. Substrate oxidation differences between high- and low-intensity exercise are compensated over 24 hours in obese men. Int. J. Obes. 2004, 28, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Melanson, E.L.; Cornier, M.A.; Bessesen, D.H.; Grunwald, G.K.; MacLean, P.S.; Hill, J.O. 24 H Metabolic Responses to Low- and High-Intensity Exercise in Lean and Obese Humans. In Proceedings of the 2005 NAASO Annual Scientific Meeting, Vancouver, BC, Canada, 15–19 October 2005; HT-01. [Google Scholar]
- Melanson, E.L.; Donahoo, W.T.; Grunwald, G.K.; Schwartz, R. Changes in 24-h substrate oxidation in older and younger men in response to exercise. J. Appl. Physiol. 2007, 103, 1576–1582. [Google Scholar] [CrossRef]
- Dionne, I.; Van Vugt, S.; Tremblay, A. Postexercise macronutrient oxidation: A factor dependent on postexercise macronutrient intake. Am. J. Clin. Nutr. 1999, 69, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Iwayama, K.; Kurihara, R.; Nabekura, Y.; Kawabuchi, R.; Park, I.; Kobayashi, M.; Ogata, H.; Kayaba, M.; Satoh, M.; Tokuyama, K. Exercise increases 24-h fat oxidation only when it is performed before breakfast. EBioMed 2015, 2, 2003–2009. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Yamamoto, Y.; Iwayama, K.; Nakamura, K.; Yamaguchi, S.; Hibi, M.; Nabekura, Y.; Tokuyama, K. Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism 2013, 62, 793–800. [Google Scholar] [CrossRef]
- Iwayama, K.; Kawabuchi, R.; Park, I.; Kurihara, R.; Kyobayashi, M.; Hibi, M.; Oishi, S.; Yasunaga, K.; Ogata, H.; Nabekura, Y.; et al. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J. Appl. Physiol. 2015, 118, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Iwayama, K.; Ogawa, A.; Tanaka, Y.; Yajima, K.; Park, I.; Ando, A.; Ogata, H.; Kayaba, M.; Zhang, S.; Tanji, F.; et al. Effects of exercise before breakfast on plasma free fatty acid profile and 24-h fat oxidation. Metabol. Open 2020, 8, 100067. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ogata, H.; Park, I.; Ando, A.; Ishihara, A.; Kayaba, M.; Yajima, K.; Suzuki, C.; Araki, A.; Osumi, H.; et al. Effect of a single bout of morning or afternoon exercise on glucose fluctuation in young healthy men. Physiol. Rep. 2021, 9, e14784. [Google Scholar] [CrossRef]
- Iwayama, K.; Kawabuchi, R.; Nabekura, Y.; Kurihara, R.; Park, I.; Kobayashi, M.; Ogata, H.; Kayaba, M.; Omi, N.; Satoh, M.; et al. Exercise before breakfast increases 24-h fat oxidation in female subjects. PLoS ONE 2017, 12, e0180472. [Google Scholar] [CrossRef] [Green Version]
- Aarts, E.; Verhage, M.; Veenvliet, J.V.; Dolan, C.V.; van der Sluis, S. A solution to dependency: Using multilevel analysis to accommo-date nested data. Nat. Neurosc. 2014, 17, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar]
- Goldstein, I.; Hager, G.L. Transcriptional and chromatin regulation during fasting-the genomic era. Trends Endocrinol. Metab. 2015, 26, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Izumida, Y.; Yahagi, N.; Takeuchi, Y.; Nishi, M.; Shikama, A.; Takarada, A.; Masuda, Y.; Kubota, M.; Matsuzaka, T.; Nakagawa, Y.; et al. Glycogen shortage during fasting triggers liver–brain–adipose neurocircuitry to facilitate fat utilization. Nat. Commun. 2013, 4, 2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philp, A.; Hargreaves, M.; Baar, K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. 2012, 302, E1343–E1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midia, M.; Odedra, D.; Shuster, A.; Midia, R.; Muir, J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: A scoping review. Fiagn. Interv. Radiol. 2019, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kamei, A.; Osawa, T.; Kawahara, T.; Takizawa, O.; Maruyama, K. 13C MRS reveals a small diurnal variation in the glycogen content of human thigh muscle. NMR Biomed. 2015, 28, 650–655. [Google Scholar] [CrossRef]
- Iwayama, K.; Onishi, T.; Maruyama, K.; Takahashi, H. Diurnal variation in the glycogen content of the human liver using 13C MRS. NMR Biomed. 2020, 33, e4289. [Google Scholar] [CrossRef]
- Iwayama, K.; Tanabe, Y.; Tanji, F.; Ohnishi, T.; Takahashi, H. Diurnal variations in muscle and liver glycogen differ depending on the timing of exercise. J. Physiol. Sci. 2021, 71, 35. [Google Scholar] [CrossRef]
- Arciero, P.J.; Ives, S.J.; Mohr, A.E.; Robinson, N.; Escudero, D.; Robinson, J.; Rose, K.; Minicucci, O.; O’Brien, G.; Curran, K.; et al. Morning exercise reduces abdominal fat and blood pressure in women; evening exercise increases muscular performance in women and lowers blood pressure in men. Front. Physiol. 2022, 13, 893783. [Google Scholar] [CrossRef]
- De Bock, K.; Derave, W.; Eijnde, B.O.; Hesselink, M.K.; Koninckx, E.; Rose, A.J.; Schrauwen, P.; Bonen, A.; Richter, E.A.; Hespel, P. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J. Appl. Physiol. 2008, 104, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Flatt, J.P. Importance of nutrient balance in body weight regulation. Diabetes Metab. Rev. 1988, 4, 571–581. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwayama, K.; Seol, J.; Tokuyama, K. Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h. Nutrients 2023, 15, 1109. https://doi.org/10.3390/nu15051109
Iwayama K, Seol J, Tokuyama K. Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h. Nutrients. 2023; 15(5):1109. https://doi.org/10.3390/nu15051109
Chicago/Turabian StyleIwayama, Kaito, Jaehoon Seol, and Kumpei Tokuyama. 2023. "Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h" Nutrients 15, no. 5: 1109. https://doi.org/10.3390/nu15051109
APA StyleIwayama, K., Seol, J., & Tokuyama, K. (2023). Exercise Timing Matters for Glycogen Metabolism and Accumulated Fat Oxidation over 24 h. Nutrients, 15(5), 1109. https://doi.org/10.3390/nu15051109