Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties
Abstract
:1. Introduction
- Do the related crossbred red rice variants reflect similar glycaemic and insulin indices (II) with UKMRC9?
- What is the relationship between nutrient content and cooking characteristics of the six rice types with the GI and II characteristics?
- Does consumption of rice with varying GI values have a role to play in modulating postprandial insulin sensitivity, pancreatic β-cell function and peptide hormones?
2. Materials and Methods
2.1. Test and Reference Food
2.2. Chemical Composition of Rice
2.3. Rice Preparation for Postprandial Testing
2.4. Subject Recruitment and Screening Procedures
2.5. Experimental Protocol
2.6. Blood Sampling, Processing and Storage Procedures
2.7. Biochemical Analyses
- [i].
- Plasma glucose: Plasma glucose concentrations (mmol/L) were quantified using a Roche Modular P800 (Roche Diagnostics, Tokyo, Japan) automated analyzer by the enzymatic hexokinase method [21]. The assay had a detection limit of 0.11 mmol/L and the intra- and inter-assay coefficients of variation (CV) were <2.0%.
- [ii].
- Plasma insulin: Heparinized plasma samples were analyzed for insulin concentrations (mU/L) using electrochemiluminescence immunoassay on the Modular Analytics E170 system (Roche Diagnostics, Tokyo, Japan). The fully-automated assay adopts a solid-phase, two-site, enzyme-labeled immunoassay based on the direct sandwich technique [22]. The intra- and inter-assay CVs were <5%, with a lower detection limit of 0.20 mU/L.
- [iii].
- Plasma lactate: The plasma L-lactate concentration (mmol/L) was assayed on a Roche Modular P800 analyser (Roche Diagnostics, Tokyo, Japan) using the lactate oxidase method [23]. The assay had a detection range between 0.22 and 15.5 mmol/L and inter-assay CV of 2.0%.
- [iv].
- Peptide hormones: Plasma concentrations of motilin (EK-045-04), neuropeptide-Y (EK-049-03) and orexin-A (EK-003-30) were determined in duplicate using commercially-available enzyme immunoassay (EIA) kits from Phoenix Pharmaceuticals (Burlingame, CA, USA), as described previously [24]. The enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s protocol and absorbance was read with a Tecan Infinite M200 microplate reader (Tecan Group Ltd., Mannedorf, Switzerland). Plasma concentrations were calculated using four-parameter non-linear logistic curve fitting (Magellan Data Analysis Software v. 311 for PC, Tecan Group Ltd., Mannedorf, Switzerland). The standard curve plots were generated using the five standard concentrations ranged from 0.01 to 100 ng/mL. The coefficients of determination for standard curves were >0.97.
2.8. Outcome Measures
- [i].
- Quality control: The mean intra-individual CV for glycaemic response after two 50 g glucose standard loads was 21.3%, which was in concordance with the recommended CV < 30% required for precision and accuracy [25].
- [ii].
- Glucometabolic markers: Kinetic markers of incremental glucose and insulin peaks are defined as maximum increases in plasma glucose and insulin concentrations obtained at any point after a test rice or glucose challenge. Incremental areas-under-the-curves (IAUC), excluding areas beneath fasting values, for plasma glucose, insulin and lactate were calculated geometrically using the trapezoidal method [19]. The GI and II were calculated by dividing the net IAUC generated from the 3 h postprandial plasma glucose-/insulin-timed responses of the test food with that by the standard glucose load (GI and II = 100), with each subject being their own reference [19]. Individual GI or II scores differing from the mean value by >2 standard deviations (outliers) were excluded from the dataset [25].
- [iii].
2.9. Statistical Analyses
3. Results
3.1. Proximate Composition and Cooking Characteristics of Rice
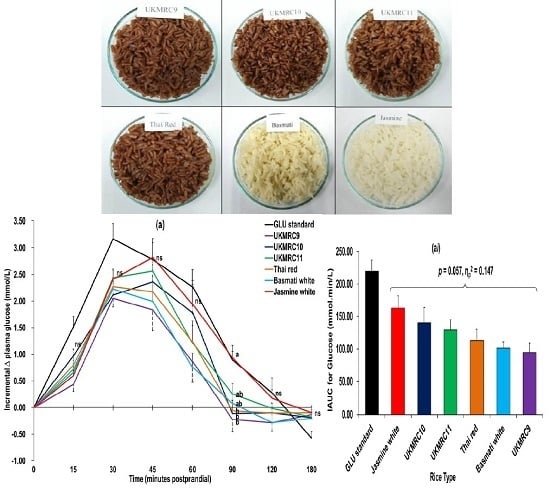
3.2. Glucometabolic Responses
3.3. Correlation between Nutrient Composition, Cooking Characteristics, GI and II
3.4. Postprandial Changes in Plasma Motilin, Neuropeptide-Y and Orexin-A
4. Discussion
4.1. Moderators of GI
4.2. Glucometabolic Responses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CV | coefficient of variation |
| EIA | enzyme immunoassay |
| FPI | fasting plasma insulin |
| GI | glycaemic index |
| GLM | general linear model |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| IAUC | incremental area-under-the-curve |
| IGI | insulinogenic index |
| II | insulin index |
| SEM | standard error of the mean |
References
- Bhullar, N.K.; Gruissem, W. Nutritional enhancement of rice for human health: The contribution of biotechnology. Biotechnol. Adv. 2013, 31, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Pan, A.; Vasanti, M.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef] [PubMed]
- The University of Sydney. GI Foods Advanced Search. Product Category: Cereal Grains. Available online: http://www.glycemicindex.com/foodSearch.php (accessed on 18 January 2016).
- Se, C.H.; Khor, B.H.; Karupaiah, T. Prospects in development of quality rice for human nutrition. Malays. Appl. Biol. 2015, 44, 1–31. [Google Scholar]
- Boers, H.M.; ten Hoorn, J.S.; Mela, D.J. A systematic review of the influence of rice characteristics and processing methods on postprandial glycaemic and insulinaemic responses. Br. J. Nutr. 2015, 114, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Kharabian-Masouleh, A.; Waters, D.L.E.; Reinke, R.F.; Ward, R.; Henry, R.J. SNP in starch biosynthesis genes associated with nutritional and functional properties of rice. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.A.; Rahman, S.; Resurreccion, A.P.; Concepcion, J.; Daygon, V.D.; Dipti, S.S.; Kabir, K.A.; Klingner, B.; Morell, M.K.; Bird, A.R. Identification of a major genetic determinant of glycaemic index in rice. Rice 2011, 4, 66–74. [Google Scholar] [CrossRef]
- Mohan, V.; Anjana, R.M.; Gayathri, R.; Bai, M.R.; Lakshmipriya, N.; Ruchi, V.L.; Balasubramaniyam, K.K.; Jakir, M.M.; Shobana, S.; Unnikrishnan, R.; et al. Glycemic index of a novel high-fiber white rice variety developed in India—A randomized control trial study. Diabetes Technol. Ther. 2016, 18, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Piao, J.H.; Tian, Y.; Li, W.D.; Li, K.J.; Yang, X.G. Postprandial glycaemic and insulinaemic responses to GM-resistant starch-enriched rice and the production of fermentation-related H2 in healthy Chinese adults. Br. J. Nutr. 2010, 103, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Zenel, A.M.; Stewart, M.L. High amylose white rice reduces post-prandial glycemic response but not appetite in humans. Nutrients 2015, 7, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Sabu, K.K.; Abdullah, M.Z.; Lim, L.S.; Wickneswari, R. Development and evaluation of advanced backcross families of rice for agronomically important traits. Commun. Biom. Crop Sci. 2006, 1, 111–123. [Google Scholar]
- Wickneswari, R.; Bhuiyan, M.A.R. The National University of Malaysia (UKM), Selangor, Malaysia. Unpublished work. 2016. [Google Scholar]
- Bhuiyan, M.A.R.; Narimah, M.K.; Rahim, H.A.; Abdullah, M.Z.; Wickneswari, R. Transgressive variants for red pericarp grain with high yield potential derived from Oryza rufipogon × Oryza sativa: Field evaluation, screening for blast disease, QTL validation and background marker analysis for agronomic traits. Field Crops Res. 2011, 121, 232–239. [Google Scholar] [CrossRef]
- Fasahat, P.; Aminah, A.; Kharidah, M.; Karupaiah, T.; Ratnam, W. Red pericarp advanced breeding lines derived from Oryza rufipogon × Oryza sativa: Physicochemical properties, total antioxidant activity, phenolic compounds and vitamin E content. Adv. J. Food Sci. Technol. 2012, 4, 155–165. [Google Scholar]
- Karupaiah, T.; Aik, C.K.; Heen, T.C.; Subramaniam, S.; Bhuiyan, A.R.; Fasahat, P.; Zain, A.M.; Ratnam, W. A transgressive brown rice mediates favourable glycaemic and insulin responses. J. Sci. Food Agric. 2011, 91, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; Lopez-Ojen, M.; Funcasta-Calderon, R.; Ameneiros-Rodriguez, E.; Donapetry-Garcia, C.; Vila-Altesor, M.; Rodriguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemistry, 16th ed.; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- FAO/WHO. Carbohydrates in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation; FAO Food and Nutrition Paper; Food and Agricultural Organization: Rome, Italy, 1998; Volume 66, pp. 1–140. [Google Scholar]
- Juliano, B.O. A simplified assay for milled-rice amylose. Cereal Sci. Today 1971, 16, 334–360. [Google Scholar]
- Schmidt, F.H. Die enzymatische bestimmung von glucose und fructose nebeneinander. Klin. Wochenschr. 1961, 39, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.M. Assays for insulin, proinsulin(s) and C-peptide. Ann. Clin. Biochem. 1999, 36, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, N.; Naka, K.; Nakajima, C.; Yoshikawa, C.; Okuda, K.; Okada, K. Test-strip method for measuring lactate in whole blood. Clin. Chem. 1989, 35, 1992–1994. [Google Scholar] [PubMed]
- Wu, H.; Xia, F.Z.; Xu, H.; Zhai, H.L.; Zhang, M.F.; Zhang, H.X.; Li, Y.X.; Li, Y.; Gu, T.; Ma, L.M.; et al. Acute effects of different glycemic index diets on serum motilin, orexin and neuropeptide Y concentrations in healthy individuals. Neuropeptides 2012, 46, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Bjorck, I.; Brighenti, F.; Brown, R.; Brynes, A.; et al. Measuring the glycemic index of foods: Interlaboratory study. Am. J. Clin. Nutr. 2008, 87, 247S–257S. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Kautzky-Willer, A.; Pacini, G. Insulinogenic indices from insulin and C-peptide: Comparison of beta-cell function from OGTT and IVGTT. Diabetes Res. Clin. Pract. 2006, 72, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, T.P.; Mallillin, A.C.; Encabo, R.R.; Sagum, R.S.; Felix, A.D.; Juliano, B.O. The effect of apparent amylose content and dietary fiber on the glycemic response of different varieties of cooked milled and brown rice. Int. J. Food Sci. Nutr. 2013, 64, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Panlasigui, L.N.; Thompson, L.U.; Juliano, B.O.; Perez, C.M.; Yiu, S.H.; Greenberg, G.R. Rice varieties with similar amylose content differ in starch digestibility and glycemic response in humans. Am. J. Clin. Nutr. 1991, 54, 871–877. [Google Scholar] [PubMed]
- Ranawana, D.V.; Henry, C.J.K.; Lightowler, H.J.; Wang, D. Glycaemic index of some commercially available rice and rice products in Great Britian. Int. J. Food Sci. Nutr. 2009, 60, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Chitrakorn, S. Rice and the Thai way of life. In Science and Technology with Thai Rice; Lorlowhakarn, S., Ed.; Thailand’s National Science and Technology Development Agency: Bangkok, Thailand, 2003; pp. 13–22. [Google Scholar]
- Yu, S.; Ma, Y.; Sun, D.W. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J. Cereal Sci. 2009, 50, 139–144. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, Q.Q.; Wilson, J.D.; Gu, M.H.; Shi, Y.C. Digestibility and physicochemical properties of rice (Oryza sativa L.) flours and starches differing in amylose content. Carbohydr. Polym. 2011, 86, 1751–1759. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Stewart, M.L. Effect of variety and cooking method on resistant starch content of white rice and subsequent postprandial glucose response and appetite in humans. Asia Pac. J. Clin. Nutr. 2013, 22, 372–379. [Google Scholar] [PubMed]
- Syahariza, Z.A.; Sar, S.; Hasjim, J.; Tizzotti, M.J.; Gilbert, R.G. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem. 2013, 136, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Juliano, B.O. Rice in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 1993. [Google Scholar]
- Dhital, S.; Dabit, L.; Zhang, B.; Flanagan, B.; Shrestha, A.K. In vitro digestibility and physicochemical properties of milled rice. Food Chem. 2015, 172, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Oztop, M.H.; Singh, R.P.; McCarthy, M.J. Physical changes in white and brown rice during simulated gastric digestion. J. Food Sci. 2011, 76, E450–E457. [Google Scholar] [CrossRef] [PubMed]
- Panlasigui, L.N.; Thompson, L.U. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int. J. Food Sci. Nutr. 2006, 57, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: Analysis of a database of more than 1000 foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Bjorck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshol, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: Similarities and differences. J. Clin. Hypertens. 2011, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, A.; Wong, J.M.W.; Mirrahimi, A.; Srichaikul, K.; Jenkins, D.J.A.; Kendall, C.W.C. Glycemic index: Physiological significance. J. Am. Coll. Nutr. 2009, 28, 439S–445S. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.C.; Coelho, R.G.; Da Silva, D.; Coelho, W.S.; Marinho-Carvalho, M.M.; Sola-Penna, M. Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett. 2011, 585, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Berhane, F.; Fite, A.; Daboul, N.; Al-Janabi, W.; Msallaty, Z.; Caruso, M.; Lewis, M.K.; Yi, Z.; Diamond, M.P.; Abou-Samra, A.B.; et al. Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J. Diabetes Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Owen, J.B.; Erickson, M.A. Insulin in the brain: There and back again. Pharmacol. Ther. 2012, 136, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Kohno, D.; Iwasaki, Y.; Yada, T. Insulin suppresses ghrelin-induced calcium signaling in neuropeptide Y neurons of the hypothalamic arcuate nucleus. Aging 2011, 3, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, T.A.; Ustione, A.; Piston, D.W. Neuropeptide Y and somatostatin inhibit insulin secretion through different mechanisms. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E211–E221. [Google Scholar] [CrossRef] [PubMed]


| Test Rice | Energy (kcal) | Total CHO (%) | Crude Protein (%) | Crude Lipid (%) | TDF (%) | Total Ash (%) | Available CHO (%) | Amylose (%) | TPC (% mg GAE) | Weight of Raw Rice (g) ‡ | Weight of Cooked Rice (g) ‡ | Cooking Time (min) § | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crossbred red rice | |||||||||||||
| UKMRC9 | 364 ± 1 a | 78.4 ± 0.10 b | 8.23 ± 0.12 a | 1.93 ± 0.26 a | 4.96 ± 0.16 a | 1.32 ± 0.01 a | 73.4 ± 0.26 c | 19.8 ± 0.35 a,b | 61.4 ± 2.59 b | 68.1 | 178.9 | 44 | |
| UKMRC10 | 355 ± 0 b,c | 76.2 ± 0.05 c | 7.44 ± 0.05 a,b | 2.20 ± 0.02 a | 4.25 ± 0.19 b | 1.30 ± 0.02 a | 71.9 ± 0.14 d | 19.0 ± 1.41 a,b | 81.7 ± 1.25 a | 69.5 | 181.1 | 45 | |
| UKMRC11 | 354 ± 0 c | 76.7 ± 0.57 c | 7.03 ± 0.55 b | 2.17 ± 0.01 a | 3.84 ± 0.14 b,c | 1.30 ± 0.03 a | 72.8 ± 0.42 c,d | 17.5 ± 0.71 b | 55.2 ± 2.03 b | 68.7 | 170.2 | 41 | |
| Commercial rice | |||||||||||||
| Thai red | 356 ± 1 b | 76.5 ± 0.20 c | 7.76 ± 0.15 a,b | 2.14 ± 0.08 a | 3.70 ± 0.09 c | 1.15 ± 0.00 b | 72.8 ± 0.30 c,d | 18.0 ± 1.41 b | 81.9 ± 3.53 a | 68.7 | 174.2 | 40 | |
| Basmati | 354 ± 1 c | 79.2 ± 0.26 a,b | 8.25 ± 0.36 a | 0.47 ± 0.10 b | 1.96 ± 0.08 d | 0.42 ± 0.01 c | 77.3 ± 0.34 b | 21.5 ± 0.71 a,b | 29.8 ± 1.60 c | 64.7 | 188.3 | 26 | |
| Jasmine | 349 ± 0 d | 79.6 ± 0.30 a | 6.98 ± 0.16 b | 0.26 ± 0.07 b | 0.24 ± 0.01 e | 0.15 ± 0.00 d | 79.4 ± 0.31 a | 23.0 ± 1.41 a | 16.2 ± 1.51 c | 62.9 | 180.3 | 32 | |
| Test Diet | GLU-Cmax (mmol/L) 1 | GLU-∆peak (mmol/L) 1 | GLU-Tmax (min) 1 | GLU-T∆0 (min) 1 | GI (%) 1 | GI Category 2 | INS-Cmax (mU/L) 1 | INS-∆peak (mU/L) 1 | IGI/HOMA-IR (×102) 1 | IGI/FPI 1 | Matsuda Index 1 | II (%) 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLU std. | 8.45 ± 0.34 | 3.43 ± 0.28 | 35.0 ± 3.4 | 121.4 ± 10.2 | 100 | - | 96.2 ± 9.96 | 90.3 ± 9.80 | 1.60 ± 0.03 | 4.91 ± 0.87 | 6.17 ± 0.64 | 100 |
| Crossbred red rice | ||||||||||||
| UKMRC9 | 7.34 ± 0.27 | 2.36 ± 0.23 | 37.5 ± 2.3 a,b | 85.8 ± 10.2 | 46 ± 7.7 | Low | 56.4 ± 5.61 | 51.5 ± 5.57 | 1.37 ± 0.03 | 4.11 ± 0.74 | 9.97 ± 0.78 | 51 ± 5.3 a |
| UKMRC10 | 8.01 ± 0.38 | 2.98 ± 0.34 | 40.0 ± 3.4 a,b | 110.2 ± 13.9 | 59 ± 8.8 | Intermediate | 63.3 ± 6.69 | 57.7 ± 6.37 | 1.41 ± 0.03 | 4.35 ± 0.98 | 8.50 ± 0.83 | 69 ± 7.7 a,b |
| UKMRC11 | 8.10 ± 0.24 | 3.08 ± 0.20 | 38.8 ± 2.2 a,b | 120.5 ± 13.8 | 63 ± 8.6 | Intermediate | 84.9 ± 10.1 | 77.1 ± 9.99 | 1.59 ± 0.04 | 4.84 ± 1.19 | 7.27 ± 0.67 | 69 ± 5.9 a,b* |
| Commercial rice | ||||||||||||
| Thai red | 7.53 ± 0.20 | 2.60 ± 0.20 | 38.8 ± 3.4 a,b | 100.5 ± 13.0 | 55 ± 8.6 | Intermediate | 67.7 ± 6.21 | 61.5 ± 5.97 | 1.43 ± 0.03 | 4.33 ± 0.85 | 8.37 ± 0.78 | 59 ± 4.0 a,b |
| Basmati | 7.37 ± 0.16 | 2.41 ± 0.12 | 35.0 ± 2.1 a | 99.6 ± 10.4 | 50 ± 5.8 | Low | 56.7 ± 4.19 | 50.9 ± 3.97 | 1.17 ± 0.02 | 3.59 ± 0.52 | 9.08 ± 0.75 | 52 ± 5.3 a,b |
| Jasmine | 8.15 ± 0.24 | 3.13 ± 0.25 | 47.5 ± 2.5 b | 136.5 ± 11.6 | 77 ± 7.3 | High | 78.7 ± 11.6 | 72.9 ± 11.6 | 1.33 ± 0.02 | 4.08 ± 0.74 | 7.04 ± 0.53 | 76 ± 7.1 b |
| p-value (ηp2) § | 0.074 ns (0.138) | 0.063 ns (0.144) | 0.043 (0.156) | 0.075 ns (0.138) | 0.093 ns (0.132) | - | 0.061 ns (0.145) | 0.069 ns (0.141) | 0.952 ns (0.017) | 0.947 ns (0.018) | 0.058 ns (0.147) | 0.018 (0.186) |
| Glycaemic Index | Insulin Index | |||
|---|---|---|---|---|
| Pearson’s r | p-Value | Pearson’s r | p-Value | |
| Rice nutrients | ||||
| Crude protein | −0.357 | 0.002 ** | −0.385 | 0.001 ** |
| Crude lipid | −0.133 | 0.268 | 0.006 | 0.958 |
| Total dietary fiber | −0.237 | 0.047 * | −0.134 | 0.263 |
| Total ash | −0.172 | 0.152 | −0.037 | 0.756 |
| Total amylose | 0.093 | 0.441 | −0.061 | 0.613 |
| Total phenolic content | −0.158 | 0.189 | −0.057 | 0.637 |
| Cooking characteristics | ||||
| Cooking time | −0.060 | 0.622 | 0.035 | 0.772 |
| Rice-to-water ratio | −0.175 | 0.145 | −0.093 | 0.442 |
| Meal serving size | −0.082 | 0.499 | −0.145 | 0.227 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Se, C.-H.; Chuah, K.-A.; Mishra, A.; Wickneswari, R.; Karupaiah, T. Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties. Nutrients 2016, 8, 308. https://doi.org/10.3390/nu8050308
Se C-H, Chuah K-A, Mishra A, Wickneswari R, Karupaiah T. Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties. Nutrients. 2016; 8(5):308. https://doi.org/10.3390/nu8050308
Chicago/Turabian StyleSe, Chee-Hee, Khun-Aik Chuah, Ankitta Mishra, Ratnam Wickneswari, and Tilakavati Karupaiah. 2016. "Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties" Nutrients 8, no. 5: 308. https://doi.org/10.3390/nu8050308
APA StyleSe, C. -H., Chuah, K. -A., Mishra, A., Wickneswari, R., & Karupaiah, T. (2016). Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties. Nutrients, 8(5), 308. https://doi.org/10.3390/nu8050308