Low Levels of Chito-Oligosaccharides Are Not Effective in Reducing Deoxynivalenol Toxicity in Swine Jejunal Explants
Abstract
:1. Introduction
2. Results
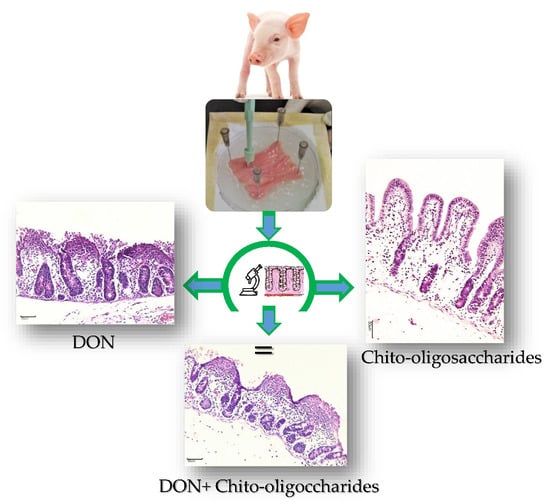
2.1. Histological Evaluation of the Explants Exposed to COS and DON
2.2. Goblet Cell Density of the Explants Exposed to COS and DON
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chito-Oligosaccharides
4.3. Culture of Explants and Exposure to DON and COS
4.4. Morphological and Morphometric Assessment
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins (Basel) 2017, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G. Effect of Processing on Mycotoxin Content in Grains. Crit. Rev. Food Sci. Nutr. 2015, 55, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Sugita-Konishi, Y.; Park, B.J.; Kobayashi-Hattori, K.; Tanaka, T.; Chonan, T.; Yoshikawa, K.; Kumagai, S. Effect of Cooking Process on the Deoxynivalenol Content and Its Subsequent Cytotoxicity in Wheat Products. Biosci. Biotechnol. Biochem. 2006, 70, 1764–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)-challenged growing pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Ghareeb, K.; Twaruzek, M.; Grajewski, J.; Bohm, J. Deoxynivalenol as a contaminant of broiler feed: Effects on bird performance and response to common vaccines. Poult. Sci. 2012, 91, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiatkiewicz, S.; Swiatkiewicz, M.; Arczewska-Wlosek, A.; Jozefiak, D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.; Hwang, J.; Kim, S.; Jeon, Y.; Je, J.; Ahn, C.; Moon, S.; Jeon, B.; Park, P. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yoo, J.S.; Kim, H.J.; Lee, J.H.; Kim, I.H. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 2009, 125, 298–303. [Google Scholar] [CrossRef]
- Maria de Souza, D.; Humberto Garcia-Cruz, C. Fermentative production of exocellular polysaccharides by bacteria. Semin. Ciênc. Agrár. 2004, 25, 331–340. [Google Scholar]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017; 157, 251–257. [Google Scholar]
- Fernandes, J.C.; Spindola, H.; De Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xing, R.; Liu, S.; Li, P. Advances in preparation, analysis and biological activities of single chitooligosaccharides. Carbohydr. Polym. 2016, 139, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Ferket, P.R.; Hong, Q.H.; Zhou, J.; Cao, G.T.; Zhou, L.; Chen, A.G. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012, 90, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S.; Thongsong, B. Effects of dietary levels of chito-oligosaccharide on ileal digestibility of nutrients, small intestinal morphology and crypt cell proliferation in weaned pigs. Livest. Sci. 2017, 198, 37–44. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan Oligosaccharide Reduces Intestinal Inflammation That Involves Calcium-Sensing Receptor (CaSR) Activation in Lipopolysaccharide (LPS)-Challenged Piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, Y.; Liu, G.; He, J.; Qiu, W.; Hu, X.; Feng, Z.; Ran, M.; Nyachoti, C.M.; Kim, S.W.; et al. Effects of chitosan on intestinal inflammation in weaned pigs challenged by enterotoxigenic Escherichia coli. PLoS ONE 2014, 9, e104192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Tang, Z.; Yin, Y.; Zhang, B.; Hu, X.; Feng, Z.; Wang, J. Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occluding, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2013, 17, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; Difilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure–activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2016, 56, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Da Silva, E.O.; Gerez, J.R.; do Carmo Drape, T.; Bracarense, A.P.F.R.L. Phytic acid decreases deoxynivalenol and fumonisin B1-induced changes on swine jejunal explants. Toxicol. Rep. 2014, 1, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. In Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Randall, K.J.; Turton, J.; Foster, J.R. Explant culture of gastrointestinal tissue: A review of methods and applications. Cell Biol. Toxicol. 2011, 27, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.F.; et al. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Buck, L.Y.; Marutani, V.H.B.; Calliari, C.M.; Cunha, L.S.; Bracarense, A.P.F.R.L. Effects of chito-oligosaccharide on piglet jejunal explants: An histological approach. Animal 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Zhou, X.L.; Lian, G.Q.; Blachier, F.; Liu, G.; Tan, B.E.; Nyachoti, C.M.; Yin, Y.L. Dietary supplementation with chitooligosaccharides alters gut microbiota and modifies intestinal luminal metabolites in weaned Huanjiang mini-piglets. Livest. Sci. 2014, 160, 97–101. [Google Scholar] [CrossRef]
- Thongsong, B.; Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S. Effects of chito-oligosaccharide supplementation with low or medium molecular weight and high degree of deacetylation on growth performance, nutrient digestibility and small intestinal morphology in weaned pigs. Livest. Sci. 2018, 209, 60–66. [Google Scholar] [CrossRef]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins (Basel) 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Gomes, F.; Bracarense, A.P.L. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in E-cadherin expression. Toxins (Basel) 2013, 5, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- De Walle, J.V.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Solís-Cruz, B.; Hernández-Patlán, D.; Beyssac, E.; Latorre, J.D.; Hernandez-Velasco, X.; Merino-Guzman, R.; Tellez, G.; López-Arellano, R. Evaluation of chitosan and cellulosic polymers as binding adsorbent materials to prevent Aflatoxin B1, Fumonisin B1, Ochratoxin, Trichothecene, Deoxynivalenol, and Zearalenone mycotoxicoses through an in vitro gastrointestinal model for poultry. Polymers (Basel) 2017, 9, 529. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Yang, L.; Wang, J.; Song, S.; Nie, D.; Yang, X.; Hou, J.; Wu, A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control 2015, 57, 362–369. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria-Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic Oligosaccharides: Special Focus on Fructooligosaccharides, Its Biosynthesis and Bioactivity. Appl. Biochem. Biotechnol. 2014, 4, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Alizadeh, A.; Verheijden, K.A.; Schoterman, M.H.; Kraneveld, A.D.; Garssen, J.; Fink-Gremmels, J. Galacto-oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J. Nutr. 2015, 145, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.; Da Silva, C.A.; Castro-Gómez, R.J.H.; Lozano, A.P.; Gavioli, D.F.; Frietzen, J.; Da Silva, E.O.; Novais, A.K.; Frederico, G.; Pereira, M. Chito-oligosaccharide as growth promoter replacement for weaned piglets: Performance, morphometry, and immune system. Semin. Agrar. 2017, 38, 3253–3269. [Google Scholar] [CrossRef]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; Di Pasquale, E.; Perrier, J.; Oswald, I.P.; Maresca, M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015, 59, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.W.; Ho, Y.C.; Chuang, E.Y.; Chen, C.T.; Juang, J.H.; Su, F.Y.; Hwang, S.M.; Sung, H.W. Effects of pH on molecular mechanisms of chitosan-integrin interactions and resulting tight-junction disruptions. Biomaterials 2013, 34, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Eijsink, V.G.H. A reliable reducing end assay for chito-oligosaccharides. Carbohydr. Polym. 2004, 56, 35–39. [Google Scholar] [CrossRef]
- Lucioli, J.; Pinton, P.; Callu, P.; Laffitte, J.; Grosjean, F.; Kolf-Clauw, M.; Oswald, I.P.; Bracarense, A.P.F.R.L. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 2013, 66, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Maidana, L.; Gerez, J.R.; El Khoury, R.; Pinho, F.; Puel, O.; Oswald, I.P.; Bracarense, A.P.F.R.L. Effects of patulin and ascladiol on porcine intestinal mucosa: An ex vivo approach. Food Chem. Toxicol. 2016, 98, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ), 0.05 mg·mL−1 of COS (50COS) (
), 0.05 mg·mL−1 of COS (50COS) (  ), DON (10 µM) (■), 25COS plus DON (25COS + DON) (
), DON (10 µM) (■), 25COS plus DON (25COS + DON) (  ), and 50COS plus DON (50COS + DON) (
), and 50COS plus DON (50COS + DON) (  ). Values are mean ± SEM. Means with unlike letters (a, b) differ significantly by Tukey’s test (p ≤ 0.05). Maximum histological score of 39 points in A.U. (arbitrary units); (b) Explants exposed to control (n = 30); (c) 25COS-exposed explant (n = 30); (d) 50COS-exposed explant (n = 30); (e) DON-exposed explant (n = 30); (f) Explant exposed to treatment 25COS + DON (n = 30); (g) Explant exposed to treatment 50COS + DON (n = 30). Histological endpoints with different arrows: simple columnar epithelium (
). Values are mean ± SEM. Means with unlike letters (a, b) differ significantly by Tukey’s test (p ≤ 0.05). Maximum histological score of 39 points in A.U. (arbitrary units); (b) Explants exposed to control (n = 30); (c) 25COS-exposed explant (n = 30); (d) 50COS-exposed explant (n = 30); (e) DON-exposed explant (n = 30); (f) Explant exposed to treatment 25COS + DON (n = 30); (g) Explant exposed to treatment 50COS + DON (n = 30). Histological endpoints with different arrows: simple columnar epithelium (  ), moderate edema of the lamina propria (†), multifocal to diffuse fusion and atrophy of villi (→), discontinuous epithelium (
), moderate edema of the lamina propria (†), multifocal to diffuse fusion and atrophy of villi (→), discontinuous epithelium (  ), necrotic debris (*), and severely flattened epithelial cells (
), necrotic debris (*), and severely flattened epithelial cells (  ) (Bar = 50 μm; Hematoxylin and eosin staining).
) (Bar = 50 μm; Hematoxylin and eosin staining).
 ), 0.05 mg·mL−1 of COS (50COS) (
), 0.05 mg·mL−1 of COS (50COS) (  ), DON (10 µM) (■), 25COS plus DON (25COS + DON) (
), DON (10 µM) (■), 25COS plus DON (25COS + DON) (  ), and 50COS plus DON (50COS + DON) (
), and 50COS plus DON (50COS + DON) (  ). Values are mean ± SEM. Means with unlike letters (a, b) differ significantly by Tukey’s test (p ≤ 0.05). Maximum histological score of 39 points in A.U. (arbitrary units); (b) Explants exposed to control (n = 30); (c) 25COS-exposed explant (n = 30); (d) 50COS-exposed explant (n = 30); (e) DON-exposed explant (n = 30); (f) Explant exposed to treatment 25COS + DON (n = 30); (g) Explant exposed to treatment 50COS + DON (n = 30). Histological endpoints with different arrows: simple columnar epithelium (
). Values are mean ± SEM. Means with unlike letters (a, b) differ significantly by Tukey’s test (p ≤ 0.05). Maximum histological score of 39 points in A.U. (arbitrary units); (b) Explants exposed to control (n = 30); (c) 25COS-exposed explant (n = 30); (d) 50COS-exposed explant (n = 30); (e) DON-exposed explant (n = 30); (f) Explant exposed to treatment 25COS + DON (n = 30); (g) Explant exposed to treatment 50COS + DON (n = 30). Histological endpoints with different arrows: simple columnar epithelium (  ), moderate edema of the lamina propria (†), multifocal to diffuse fusion and atrophy of villi (→), discontinuous epithelium (
), moderate edema of the lamina propria (†), multifocal to diffuse fusion and atrophy of villi (→), discontinuous epithelium (  ), necrotic debris (*), and severely flattened epithelial cells (
), necrotic debris (*), and severely flattened epithelial cells (  ) (Bar = 50 μm; Hematoxylin and eosin staining).
) (Bar = 50 μm; Hematoxylin and eosin staining).
 ), 0.05 mg·mL−1 of COS (50COS) (
), 0.05 mg·mL−1 of COS (50COS) (  ), DON (10 µM) (■), 25COS plus DON (25COS + DON) (
), DON (10 µM) (■), 25COS plus DON (25COS + DON) (  ), and 50COS plus DON (50COS + DON) (
), and 50COS plus DON (50COS + DON) (  ). Values are mean ± SEM represented by vertical bars. Means with unlike letters (a, b) differ significantly by Tukey’s test (p < 0.05).
). Values are mean ± SEM represented by vertical bars. Means with unlike letters (a, b) differ significantly by Tukey’s test (p < 0.05).
 ), 0.05 mg·mL−1 of COS (50COS) (
), 0.05 mg·mL−1 of COS (50COS) (  ), DON (10 µM) (■), 25COS plus DON (25COS + DON) (
), DON (10 µM) (■), 25COS plus DON (25COS + DON) (  ), and 50COS plus DON (50COS + DON) (
), and 50COS plus DON (50COS + DON) (  ). Values are mean ± SEM represented by vertical bars. Means with unlike letters (a, b) differ significantly by Tukey’s test (p < 0.05).
). Values are mean ± SEM represented by vertical bars. Means with unlike letters (a, b) differ significantly by Tukey’s test (p < 0.05).
| Treatments | Villi Height, µm | Crypt Depth, µm | Villi Height:Crypt Depth, μm:μm |
|---|---|---|---|
| Control | 139.68 ± 8.48 a | 129.21 ± 8.53 a | 1.09 ± 0.07 a |
| 25COS | 121.74 ± 10.50 ab | 136.69 ± 16.11 a | 0.90 ± 0.05 ab |
| 50COS | 116.56 ± 13.71 ab | 110.77 ± 3.34 a | 1.04 ± 0.09 a |
| DON | 87.60 ± 4.01 b | 140.86 ± 18.60 a | 0.64 ± 0.06 b |
| 25COS + DON | 81.79 ± 9.99 b | 131.09 ± 8.18 a | 0.63 ± 0.09 b |
| 50COS + DON | 86.78 ± 7.50 b | 132.34 ± 13.39 a | 0.65 ± 0.02 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerez, J.; Buck, L.; Marutani, V.H.; Calliari, C.M.; Bracarense, A.P. Low Levels of Chito-Oligosaccharides Are Not Effective in Reducing Deoxynivalenol Toxicity in Swine Jejunal Explants. Toxins 2018, 10, 276. https://doi.org/10.3390/toxins10070276
Gerez J, Buck L, Marutani VH, Calliari CM, Bracarense AP. Low Levels of Chito-Oligosaccharides Are Not Effective in Reducing Deoxynivalenol Toxicity in Swine Jejunal Explants. Toxins. 2018; 10(7):276. https://doi.org/10.3390/toxins10070276
Chicago/Turabian StyleGerez, Juliana, Letícia Buck, Victor Hugo Marutani, Caroline Maria Calliari, and Ana Paula Bracarense. 2018. "Low Levels of Chito-Oligosaccharides Are Not Effective in Reducing Deoxynivalenol Toxicity in Swine Jejunal Explants" Toxins 10, no. 7: 276. https://doi.org/10.3390/toxins10070276
APA StyleGerez, J., Buck, L., Marutani, V. H., Calliari, C. M., & Bracarense, A. P. (2018). Low Levels of Chito-Oligosaccharides Are Not Effective in Reducing Deoxynivalenol Toxicity in Swine Jejunal Explants. Toxins, 10(7), 276. https://doi.org/10.3390/toxins10070276