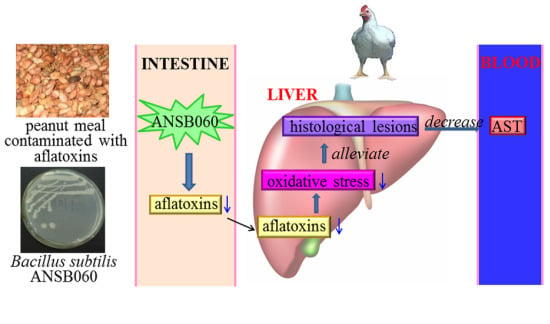
1. Introduction
Aflatoxins are a class of potent mycotoxins produced mainly by
Aspergillus flavus,
Aspergillus parasiticus, and occasionally by other
Aspergillus species [
1]. Aflatoxins occur widely in naturally contaminated animal feeds and human foods, such as peanuts, maize, distiller’s dried grains with solubles (DDGS), silage, oilseeds, milk, cheese, fruit juice, and other agricultural feed or food [
2,
3]. Aflatoxins constitute a great threat to the health of animals and humans due to their teratogenic, carcinogenic, mutagenic, and immunosuppressive effects [
4,
5]. Additionally, in terms of the livestock industry, aflatoxins cause huge economic loss by retarding animal growth, increasing feed consumption, and reducing meat production [
6,
7]. Among the various types of aflatoxins, aflatoxin B
1 (AFB
1) is known to be the most biologically active component.
It is reported that the liver is the main target organ for aflatoxins. Long-term intake of feeds contaminated with aflatoxins results in negative effects on the liver, such as hepatic cell and tissue injury [
6], as well as gross and microscopic abnormalities [
8]. Abdel-Wahhab and Aly [
9] reported that aflatoxins caused membrane damage in rat liver through increased lipid peroxidation. According to Özen
et al. [
10], AFB
1 increased the malondialdehyde (MDA) level, and induced vacuolar degeneration, necrosis, and bile duct hyperplasia in chicken liver. These deteriorations were confirmed to increase susceptibility to infectious disease and mortality in animals [
11]. Moreover, residues of aflatoxins in the tissues, milk, and eggs of animals are a potential hazard to human health [
12]. Researchers revealed the relationship between aflatoxin exposure and high incidence of human liver cancer in various areas of Asia and Africa [
13]. Aflatoxins can act synergistically with the hepatitis B virus to enhance the risk of hepatocellular carcinoma [
14]. Since the occurrence of aflatoxins in feeds and foods is generally at low concentrations [
2,
3], there is interest in studying the effects of long-term exposure to low levels of aflatoxins on animals and humans.
Numerous physical, chemical and biological methods have been proposed to detoxify or inactivate aflatoxins in contaminated feedstuffs. Among them, the use of biological methods, using microorganisms and their metabolites to eliminate aflatoxins, can be a highly promising approach owing to its specific, efficient, and environmentally sound detoxification [
15]. Some microbes, including fungal and bacterial isolates, such as
Flavobacterium aurantiacum [
16],
Stenotrophomonas Maltophilia [
5],
Myxococcus fulvus [
17], and
Aspergillus niger [
18], were reported to effectively biodegrade aflatoxins
in vitro. However, little is known about their efficiency in the biodegradation of aflatoxins and effect on aflatoxicosis
in vivo. Our lab screened a strain of probiotic bacteria
Bacillus subtilis ANSB060 from the fish gut, which can be directly applied in the feedstuffs and feeds [
19]. This strain possessed strong degradation ability against aflatoxins (up to 81.5%). Particularly, the cell-free culture supernatant showed effective aflatoxin-degrading activity (up to 78.7%), suggesting that
B. subtilis ANSB060 can detoxify aflatoxins via biotransformation rather than binding or absorbing aflatoxins to cell walls. In addition, it exhibited antimicrobial activity and high resistance to the simulated gut environment. Because
B. subtilis is generally recognized as safe, it is feasible for
B. subtilis ANSB060 to be applied in the feeds and test its protective effects against aflatoxicosis in animals. The effects of supplementation of
B. subtilis ANSB060 in the aflatoxin-contaminated diets on improving growth performance in layers and broilers have been verified well in our previous studies [
7,
20]. However, the role of dietary
B. subtilis ANSB060 in protecting hepatic structure and function from aflatoxins in broilers has not been assessed so far.
The objective of this study was to examine the toxic effect of aflatoxins and protective efficacy of B. subtilis ANSB060 on serum biochemistry, liver histopathology, serum and liver antioxidant enzymes activities, and aflatoxin concentrations in the duodenal content of broilers exposed to feed naturally contaminated with aflatoxins.
2. Results
2.1. Determination of Mycotoxin Content
The naturally moldy peanut meal used in the diets was mainly contaminated with aflatoxins. The concentrations of AFB1, AFB2, AFG1 and AFG2 were determined to be 70.7 ± 1.3, 11.0 ± 1.5, 6.5 ± 0.8 and 2.0 ± 0.3 µg/kg diet, respectively, in the treatment diets of M0, M0.5, M1.0 and M2.0. The content of zearalenone (ZEA) in the moldy diets (M0, M0.5, M1.0 and M2.0) was only 4.1 ± 0.3 µg/kg diet. Other mycotoxins, namely deoxynivalenol (DON) and ochratoxin A (OTA), were not detected in the diets of M0, M0.5, M1.0 and M2.0. The concentrations of all mycotoxins analyzed in the diets of C0 and C1.0 were below the detection limits under current analytical conditions. The detection limits for AFB1, AFB2, AFG1, AFG2, DON, ZEA and OTA were 1.0, 0.7, 3.7, 1.8, 60, 3.0 and 0.5 µg/kg diet, respectively.
2.2. Organ Weights and Serum Biochemistry
No significant differences were observed for the weights of organs (heart, liver, spleen, bursa of fabricius and thymus) among treatments (
p > 0.05;
Table 1). However, the aspartate aminotransferase (AST) activity measured in the chickens fed diet M0 was increased by 37% (
p < 0.001;
Table 2) as compared with the chickens fed diet C0. The AST activity in group C1.0 was not different from group C0 (
p > 0.05). Supplementation of
B. subtilis ANSB060 to diets contaminated with aflatoxins decreased the AST activity and the decrease magnitude was associated with the level of
B. subtilis ANSB060 supplemented. Polynomial orthogonal contrasts showed that both linear and quadratic responses to
B. subtilis ANSB060 supplementation in the aflatoxin-contaminated diet were significant (
p < 0.001). The AST activity measured in chickens fed diet M2.0 had no difference from diet C0. No differences were detected for all the other enzymes among the treatment groups (
p > 0.05).
Table 1.
Effects of B. subtilis ANSB060 on the relative organ weights (g/kg of body weight (BW)) of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
Table 1.
Effects of B. subtilis ANSB060 on the relative organ weights (g/kg of body weight (BW)) of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
| Item | C0 | C1.0 | M0 | M0.5 | M1.0 | M2.0 | SEM | p-Value |
|---|
| F-test | Linear | Quadratic |
|---|
| BW, kg | 2.353 a | 2.344 a | 2.257 b | 2.294 ab | 2.320 ab | 2.331 a | 0.010 | 0.049 | 0.019 | 0.571 |
| Heart | 3.33 | 3.15 | 3.22 | 3.10 | 3.30 | 3.14 | 0.113 | 0.652 | 0.914 | 0.853 |
| Liver | 22.90 | 22.80 | 23.93 | 23.23 | 23.52 | 23.76 | 0.618 | 0.749 | 0.937 | 0.456 |
| Spleen | 1.08 | 1.20 | 1.11 | 1.14 | 1.22 | 1.16 | 0.073 | 0.790 | 0.499 | 0.544 |
| Bursa of fabricius | 0.51 | 0.54 | 0.61 | 0.62 | 0.61 | 0.57 | 0.038 | 0.195 | 0.417 | 0.518 |
| Thymus | 1.45 | 1.35 | 1.72 | 1.56 | 1.83 | 1.74 | 0.203 | 0.541 | 0.722 | 0.878 |
Table 2.
Effects of B. subtilis ANSB060 on serum biochemical parameters of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
Table 2.
Effects of B. subtilis ANSB060 on serum biochemical parameters of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
| Item | C0 | C1.0 | M0 | M0.5 | M1.0 | M2.0 | SEM | p-Value |
|---|
| F-test | Linear | Quadratic |
|---|
| TP, g/L | 25.88 | 25.25 | 23.87 | 23.74 | 23.91 | 24.04 | 0.968 | 0.524 | 0.876 | 0.894 |
| ALT, U/L | 2.08 | 2.20 | 1.97 | 2.19 | 2.03 | 1.83 | 0.423 | 0.990 | 0.766 | 0.611 |
| AST *, U/L | 34.25 b | 36.22 ab | 47.14 a | 48.29 a | 41.45 ab | 31.84 b | 0.853 | <0.001 | <0.001 | <0.001 |
| ALP, U/L | 1419.5 | 1425.8 | 1517.0 | 1489.4 | 1440.4 | 1437.6 | 49.291 | 0.678 | 0.203 | 0.803 |
2.3. Serum and Liver Antioxidant Enzyme Activities
Serum and hepatic total superoxide dismutase (SOD) activities were not affected (p > 0.05) by dietary treatments.
The serum activity of glutathione peroxidase (GSH-Px) measured in chickens under M0 treatment was on average 7% lower (
p < 0.05) than that in the chickens under C0 and C1.0 treatments but was enhanced with the supplementation of
B. subtilis ANSB060 at the rate of 2 g/kg of M0 diet (
Table 3). This enhancement was linear with the increase of
B. subtilis ANSB060 supplemented in the moldy diets (
p < 0.05). No difference in GSH-Px activity was detected between group M2.0 and C0. The serum MDA content was increased by 46% on average in chickens fed diet M0 compared with the chickens fed diets C0 and C1.0 (
p < 0.001). Supplementation of
B. subtilis ANSB060 at 2 g/kg of M0 diet decreased serum MDA content. A negative linear response of serum MDA level to the amount of
B. subtilis ANSB060 was also observed (
p = 0.001). No difference was tested in the serum MDA between chickens fed diets M2.0 and C0.
Like the serum MDA level, the level of liver MDA in group M0 was on average 18% higher (p < 0.05) than that in C0 and C1.0, and supplementing B. subtilis ANSB060 to the moldy diets restored the MDA content under treatment M2.0. The hepatic MDA content declined linearly (p < 0.05) with increase in the levels of B. subtilis ANSB060. Chickens fed diets of M1.0 and M2.0 had similar MDA content in the liver (p > 0.05) as chickens fed a diet of C0.
Table 3.
Effects of B. subtilis ANSB060 on serum and liver antioxidant index of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
Table 3.
Effects of B. subtilis ANSB060 on serum and liver antioxidant index of broilers fed moldy peanut meal naturally contaminated with aflatoxins 1.
| Item | C0 | C1.0 | M0 | M0.5 | M1.0 | M2.0 | SEM | p-Value |
|---|
| F-test | Linear | Quadratic |
|---|
| Serum | - | - | - | - | - | - | - | - | - | - |
| SOD, U/mgprot | 119.33 | 116.01 | 110.86 | 113.19 | 115.01 | 117.75 | 2.943 | 0.404 | 0.093 | 0.943 |
| GSH-Px, U/mgprot | 491.62 a | 492.04 a | 457.25 c | 468.12 bc | 469.56 bc | 479.75 ab | 6.686 | 0.005 | 0.027 | 0.960 |
| MDA *, nmol/mgprot | 16.01 c | 17.11 c | 24.21 a | 22.64 a | 21.14 ab | 18.63 bc | 1.025 | <0.001 | 0.001 | 0.656 |
| Liver | - | - | - | - | - | - | - | - | - | - |
| SOD *, U/mgprot | 297.15 | 296.83 | 275.48 | 293.11 | 291.32 | 293.66 | 6.899 | 0.332 | 0.106 | 0.280 |
| GSH-Px, U/mgprot | 72.483 | 72.339 | 63.157 | 67.315 | 67.640 | 71.783 | 2.589 | 0.095 | 0.031 | 0.997 |
| MDA *, nmol/mgprot | 0.218 c | 0.225 bc | 0.262 a | 0.245 ab | 0.240 abc | 0.227 bc | 0.008 | 0.007 | 0.004 | 0.824 |
2.4. Histopathology
Macroscopically, the livers from the birds fed a diet of M0 were slightly enlarged and pale in color compared to those from the birds under the control treatment (
Figure 1). The livers of birds in group C1.0 had an appearance similar to those in group C0. Supplementation of B. subtilis ANSB060 in the diets containing aflatoxins could ameliorate these changes. As presented in
Figure 1, the intensity of the amelioration appeared to increase in a dose related manner.
Figure 2 shows the photomicrographs of hematoxylin and eosin-stained liver sections of the birds from different dietary treatments. There were no visible lesions in the livers of the birds from groups C0 and C1.0. Livers from the birds consuming diet M0 showed significant lesions such as bile duct epithelium hyperplasia, vacuolar degeneration in hepatocytes, and lymphocyte infiltration in hepatocytes and portal tract. The supplementation of
B. subtilis ANSB060 to diet M0 partially decreased the severity of these lesions. As the amount of
B. subtilis ANSB060 increased in the diet, the amelioration on the lesions was more apparent, especially under the M2.0 treatment.
Figure 1.
Representative livers from broilers (42 days old) fed different diets. C0, the negative control diet; C1.0, the negative control diet plus 1.0 g B. subtilis ANSB060 /kg diet; M0, the aflatoxin-contaminated diet; M0.5, the aflatoxin-contaminated diet plus 0.5 g B. subtilis ANSB060/kg diet; M1.0, the aflatoxin-contaminated diet plus 1.0 g B. subtilis ANSB060/kg diet; M2.0, the aflatoxin-contaminated diet plus 2.0 g B. subtilis ANSB060/kg diet.
Figure 1.
Representative livers from broilers (42 days old) fed different diets. C0, the negative control diet; C1.0, the negative control diet plus 1.0 g B. subtilis ANSB060 /kg diet; M0, the aflatoxin-contaminated diet; M0.5, the aflatoxin-contaminated diet plus 0.5 g B. subtilis ANSB060/kg diet; M1.0, the aflatoxin-contaminated diet plus 1.0 g B. subtilis ANSB060/kg diet; M2.0, the aflatoxin-contaminated diet plus 2.0 g B. subtilis ANSB060/kg diet.
Figure 2.
Representative photomicrographs (optical microscopy) of hematoxylin and eosin-stained broiler liver sections from different treatments. (C0) normal histological structure of liver lobule, central vein and hepatocytes are observed in broilers fed negative control diet; (C1.0) normal hepatocytes are present in broilers fed negative control diet plus 1.0 g B. subtilis ANSB060 /kg diet; (M0) obvious liver lesions such as bile duct epithelium hyperplasia (blue arrow), lymphocyte infiltration in hepatocytes and portal tract (black arrow), and vacuolar degeneration in hepatocytes (red arrow) are observed in broilers fed diet contaminated with aflatoxins; (M0.5) lymphocyte infiltration in hepatocytes (black arrow) and less vacuolar degeneration in hepatocytes (red arrow) are present in broilers fed aflatoxin-contaminated diet plus 0.5 g B. subtilis ANSB060/kg diet; (M1.0) less lymphocyte infiltration in hepatocytes (black arrow) is observed in broilers fed aflatoxin-contaminated diet plus 1.0 g B. subtilis ANSB060/kg diet; (M2.0) the least lymphocyte infiltration in hepatocytes (black arrow) is present in broilers fed aflatoxin-contaminated diet plus 2.0 g B. subtilis ANSB060/kg diet. Scale bar = 50 μm.
Figure 2.
Representative photomicrographs (optical microscopy) of hematoxylin and eosin-stained broiler liver sections from different treatments. (C0) normal histological structure of liver lobule, central vein and hepatocytes are observed in broilers fed negative control diet; (C1.0) normal hepatocytes are present in broilers fed negative control diet plus 1.0 g B. subtilis ANSB060 /kg diet; (M0) obvious liver lesions such as bile duct epithelium hyperplasia (blue arrow), lymphocyte infiltration in hepatocytes and portal tract (black arrow), and vacuolar degeneration in hepatocytes (red arrow) are observed in broilers fed diet contaminated with aflatoxins; (M0.5) lymphocyte infiltration in hepatocytes (black arrow) and less vacuolar degeneration in hepatocytes (red arrow) are present in broilers fed aflatoxin-contaminated diet plus 0.5 g B. subtilis ANSB060/kg diet; (M1.0) less lymphocyte infiltration in hepatocytes (black arrow) is observed in broilers fed aflatoxin-contaminated diet plus 1.0 g B. subtilis ANSB060/kg diet; (M2.0) the least lymphocyte infiltration in hepatocytes (black arrow) is present in broilers fed aflatoxin-contaminated diet plus 2.0 g B. subtilis ANSB060/kg diet. Scale bar = 50 μm.
![Toxins 07 03330 g002]()
2.5. Levels of Aflatoxins Recovered from Duodenal Content
The amounts of aflatoxins recovered from duodenal content are given in
Figure 3. Neither AFG
1 nor AFG
2 was detected in duodenal contents (detection limit, 1.00 ng/g for AFG
1 and 0.50 ng/g for AFG
2). AFB
1 and AFB
2 were not detected in the duodenum of broilers fed diets C0 and C1.0 (detection limit, 0.30 ng/g for AFB
1 and 0.20 ng/g for AFB
2). Among other treatments, AFB
1 level was the highest in group M0 at 7.21 ng/g freeze-dried matter. Compared to group M0, supplementing
B. subtilis ANSB060 to contaminated diets (M0.5, M1.0 and M2.0) markedly reduced the AFB
1 concentration by 46.05% (3.89 ng/g), 67.13% (2.37 ng/g) and 76.70% (1.68 ng/g), respectively. The amount of AFB
2 in all groups had a similar trend as that of AFB
1. The level of AFB
2 in group M0 was at 1.94 ng/g, and reduced by 43.81% (1.09 ng/g), 62.37% (0.73 ng/g) and 79.90% (0.39 ng/g) (
p < 0.05) with the increase of supplementing
B. subtilis ANSB060 to groups M0.5, M1.0, and M2.0, respectively. Both the AFB
1 and AFB
2 concentrations showed linear and quadratic responses to the increasing addition of
B. subtilis ANSB060 to the M0 diet.
Figure 3.
Effects of B. subtilis ANSB060 on AFB1 (a) and AFB2 (b) recovered from duodenal content of broilers fed moldy peanut meal naturally contaminated with aflatoxins. Values are expressed as mean ± SE, n = 6. Columns with different letters are significantly different (p < 0.05). * AFB1 and AFB2 were not detected in the duodenum of broilers under C0 and C1.0 treatments; AFB1 and AFB2 showed linear and quadratic responses to the increasing addition of B. subtilis ANSB060 in the moldy diet (p < 0.05).
Figure 3.
Effects of B. subtilis ANSB060 on AFB1 (a) and AFB2 (b) recovered from duodenal content of broilers fed moldy peanut meal naturally contaminated with aflatoxins. Values are expressed as mean ± SE, n = 6. Columns with different letters are significantly different (p < 0.05). * AFB1 and AFB2 were not detected in the duodenum of broilers under C0 and C1.0 treatments; AFB1 and AFB2 showed linear and quadratic responses to the increasing addition of B. subtilis ANSB060 in the moldy diet (p < 0.05).
4. Experimental Section
4.1. Animals, Design and Diets
A total of 360 one-day-old male broiler chickens (Ross 308) were purchased from a commercial hatchery. After an acclimatization period of seven days, broilers (body weight = 162.0 ± 0.4 g) were assigned randomly into six treatments with six replicates per treatment and ten birds per replicate. The diets for the treatments were: C0 (the negative control, a basal diet containing 21% normal peanut meal); C1.0 (the negative control supplemented with 1.0 g
B. subtilis ANSB060/kg diet); M0 (the positive control, the basal diet containing 21% moldy peanut meal substituting for the normal peanut meal); and M0.5, M1.0 and M2.0 (the positive control supplemented with 0.5, 1.0 and 2.0 g
B. subtilis ANSB060/kg diet). The basal diet (
Table 4) was formulated to meet the nutrient requirements of the National Research Council (1994). After preparing the diet, two samples of feed from each treatment were analyzed to ensure mycotoxin concentrations in the experimental diets. The concentrations of aflatoxins (including AFB
1, AFB
2, AFG
1 and AFG
2), DON, ZEA and OTA in the diets were determined using high performance liquid chromatography (HPLC) as described by Binder
et al. [
2]. Briefly, 25 g of milled samples were well-mixed with 100 mL of methanol-water (80:20, vol/vol) for aflatoxins; water for DON; acetonitrile-water (70:30, vol/vol) for ZEA; methanol-water (60:40, vol/vol) for OTA. The mixtures were shaken vigorously for 1 h. The extract was filtered, and the filtrate was cleaned up through an immunoaffinity column (Vicam, Milford, MA, USA) before HPLC (Shimadzu LC-10 AT, Shimadzu, Tokyo, Japan) determination. An aflatoxin biodegradation preparation consisting mainly of
B. subtilis ANSB060 was produced by industrial fermentation and dry-processing technologies. The viable count of
B. subtilis ANSB060 in the aflatoxin biodegradation preparation was more than 1 × 10
9 CFU/g.
Table 4.
Basal diet formulations and nutritional contents.
Table 4.
Basal diet formulations and nutritional contents.
| Ingredients | Percentage (%) | Nutrition component | Content |
|---|
| Maize | 57.70 | Crude protein, % | 21.48 |
| Extruded-soybean | 6.00 | Metabolisable energy, MJ/kg | 12.60 |
| Soybean meal | 8.20 | Calcium, % | 0.99 |
| Peanut meal | 21.00 | Total phosphorus, % | 0.65 |
| Limestone | 1.37 | Available phosphorus, % | 0.43 |
| Calcium hydrophosphate | 1.80 | Methionine, % | 0.62 |
| Salt | 0.30 | Methionine + Cystine, % | 0.91 |
| Soybean oil | 2.00 | Lysine, % | 1.15 |
| Lysine [98.5%] | 0.47 | Tryptophan, % | 0.21 |
| DL-methionine | 0.36 | Threonine, % | 0.81 |
| Threonine | 0.19 | - | - |
| Vitamin premix 1 | 0.03 | - | - |
| Choline chloride | 0.10 | - | - |
| Mineral premix 2 | 0.30 | - | - |
| Zeolite powder | 0.18 | - | - |
| Total | 100.00 | - | - |
The broilers were given humane care in compliance with the guidelines of the Animal Welfare Committee of China Agricultural University. All broilers were raised in wire cages in a three-level battery. Broilers were exposed to 24-h continuous lighting for the first three days and 23L: 1D (23 h of light and 1 h of darkness) from four days of age onward. The temperature was initially maintained at 30 °C for the first week and gradually decreased to 21 °C until 24 days and maintained at 21 °C thereafter. The relative humidity was maintained at between 65% and 70%. Ventilation was controlled by negative pressure using fans. Feed and water were provided ad libitum via tube feeders and nipple drinkers during the entire experimental period.
4.2. Serum Biochemistry and Organ Weights
At 42 days of age, two birds close to the average weight were selected from each replicate. After the birds were fasted for 12 h, blood samples were collected in tubes without anticoagulant by puncture of the wing vein. The samples were centrifuged at 1000× g at 4 °C for 10 min, and the serum was separated and stored at −70 °C until biochemical analysis. The TP content, along with the ALT, AST, and ALP activities were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) with the colorimetric method following the kit instructions. After taking blood samples, the birds were euthanized humanely by cervical dislocation, and the liver, heart, spleen, bursa of fabricius, and thymus were removed and weighed immediately.
4.3. Serum and Liver Antioxidant Enzyme Activities Assays
Within 1 h postmortem, the liver samples (the tip of the left lobe) were removed and washed in ice-cold physiological saline. Approximately 0.5 g of liver was homogenized in 4.5 mL ice-cold physiological saline using an Ultra-Turrax (T8, IKA-Labortechnik, Staufen, Germany). The homogenate was then centrifuged at 1200× g at 4 °C for 10 min. The supernatant was collected and stored in a freezer at −70 °C for the following analysis. The activities of SOD and GSH-Px, and contents of MDA in the serum and hepatic supernatants were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the kit instructions.
4.4. Histopathological Measurements
Part of the liver sample (the tip of the right lobe) from the birds was also taken and fixed in 10% neutral-buffered formalin solution. The fixed tissue was dehydrated in graded alcohol, cleared in xylene, and embedded in paraffin. Thin sections (3 to 5 μm) were sliced and then stained with hematoxylin and eosin for histopathological examination using optical microscopy (Olympus Optical Company, Tokyo, Japan) as described by Bancroft and Gamble [
31].
4.5. Aflatoxins Recovered from Duodenal Content Measurements
At 42 days of age, another two birds (without fasting) with the average body weight in each replicate were selected and euthanized humanely by cervical dislocation. The duodenal contents (from ventriculus to pancreo-biliary ducts) of the two birds from the same replicate were collected in the same tube. To remove all the content, the duodenum was rinsed with 15 mL phosphate buffered saline (PBS, pH = 7.4).
The analysis of aflatoxin levels recovered from duodenum was carried out according to the methods of the Association of Official Analytical Chemists (AOAC, 2000). The sample was cleaned up before HPLC determination. Specifically, 0.5 g of the freeze-dried duodenal contents were blended in 4 mL of methanol-water (80:20, vol/vol) for 3 min, and the resulted homogenate was centrifuged at 2500× g for 5 min. The supernatant (2 mL) obtained from the centrifugation was diluted with 8 mL of PBS and passed through an immunoaffinity column (Vicam, Milford, MA, USA). Aflatoxins were eluted from the column with 1.0 mL methanol into a clear glass tube, and the methanol was evaporated to dryness under a gentle stream of nitrogen. The residue was dissolved in the HPLC mobile phase for analysis. The HPLC system (Shimadzu LC-10 AT, Shimadzu, Tokyo, Japan) was equipped with a reverse phase column (DIKMA, C18, 5 μm, 15 cm × 4.6 cm ID), a post-column photochemical derivation (Aura Industries, Staten Island, NY, USA), and a fluorescence monitor (Shimadzu RF-20A, Shimadzu, Tokyo, Japan). The wavelengths of fluorescence detection were 360 nm for excitation and 440 nm for emission. The mobile phase was methanol-water (45:55, vol/vol), and the flow rate was 1 mL/min.
4.6. Statistical Analysis
All data were analyzed according to a completely randomized experimental design, using the GLM procedure of SAS software (Version 9; SAS Institute, Inc., Cary, NC, USA). Duncan’s multiple range test was used for multiple comparisons when a significant difference was detected. Contrasts were performed to test the difference between the means of the moldy diet without and with B. subtilis ANSB060 (M0 vs. M0.5+M1.0+M2.0). Polynomial orthogonal contrasts were used to determine linear and quadratic responses to B. subtilis ANSB060 supplementation in the moldy diet. All statements of significance were based on the <0.05 level of probability.