Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications
Abstract
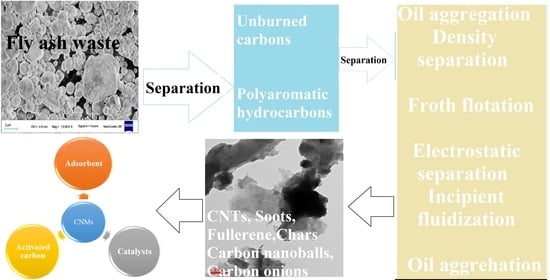
:1. Introduction
2. Importance of Carbon Nanomaterial and PAHs
3. Polycyclic Aromatic Hydrocarbons (PAHs) Presence in CFA
4. CFA as a Natural Source of Carbon Nanomaterials (CNMs) and PAHs
4.1. CFA as a Natural Source of CNMs
4.2. Coal Fly Ash as a Source Material of PAHs
5. Estimation of Carbon Content in CFA
6. Recovery of Carbon Nanomaterial from CFA
6.1. Wet Separation Method
6.1.1. Froth Flotation (FF)
6.1.2. Oil Agglomeration
6.1.3. Density Separation (Sink-Float Technique)
6.2. Dry Separation Method
6.2.1. Separation by Size Classification
6.2.2. Electrostatic Separation (ES)
6.2.3. Incipient Fluidization
6.2.4. Tribo-Electrostatic Separation (TES)
7. Types of Carbon Nanomaterial Present in CFA
7.1. Fullerene
Fullerene and Fullerene-Like Materials in CFA
7.2. Nanocarbon and Nanocoating
7.3. Carbon Nanotubes and Their Properties in CFA
7.4. Carbon Nanoballs
7.5. Carbon Onions
7.6. Extraction of Soot and Chars from CFA
7.6.1. Chars
7.6.2. Soot
8. Properties of Carbon Nanomaterials from CFA
9. PAHs in CFA and Bottom Ash
9.1. Formation of PAHs in Coal
9.2. Extraction Method of PAHs from CFA
9.3. Methods for Analysis of PAHs
9.4. Properties of PAHs Extracted from CFA
10. Applications of CNMs, Unburned Carbon, and PAHs of CFA
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCNTs | Branched carbon nanotubes |
| BD | Bulk density |
| BPAA | Bubble–particle attachment angle |
| CEA | Central electrical agency |
| CF | Calorific value |
| CFA | Coal fly ash |
| CNFs | Carbon nano-fullerenes |
| CNMs | Carbon nanomaterials |
| CNTS | Carbon nanotubes |
| ED | Envelope density |
| EDS | Electron diffraction spectroscopy |
| EELS | Electron energy loss spectroscopy |
| ESCA | Electron scattering chemical analysis |
| ESP | Electrostatic precipitator |
| FA | Fly ash |
| FF | Froth flotation |
| GC | Gas chromatography |
| GCMS | Gas chromatography mass spectroscopy |
| HCFA | High carbon fly ash |
| HRTEM | High-resolution transmission scanning electron microscopy |
| IEA | International energy authority |
| LOI | Loss on ignition |
| MTs | Million tons |
| MWCNTs | Multi-walled carbon nanotubes |
| NPs | Nanoparticles |
| PAHs | Polyaromatic hydrocarbons |
| PCHs | Polycyclic hydrocarbons |
| PM | Particulate matters |
| POPs | Persistent organic pollutants |
| SD | Skeleton density |
| SEM | Scanning electron microscope |
| SFT | Sink-float technique |
| STEM | Scanning tunneling electron microscope |
| SVR | Surface area-to-volume ratio |
| SWCNTs | Single walled carbon nanotubes |
| TEM | Transmission electron microscopy |
| TES | Tribo-electrostatic separator |
| TGA | Thermo gravimetric analysis |
| TOC | Total organic carbon |
| TPPs | Thermal power plants |
| UC | Unburned carbon |
| XPS | X-ray photoelectron spectroscopy |
References
- Yadav, V.K.; Fulekar, M.H. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics 2020, 3, 384–420. [Google Scholar] [CrossRef]
- Vassilev, S.; Menendez, R.; Alvarez, D.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 1. Characterization of Feed Coals and Fly Ashes. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Vassilev, S.; Menendez, R.; Borrego, A.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 2004, 83, 1563–1583. [Google Scholar] [CrossRef]
- Yadav, V.K.; Pandita, P.R. Fly Ash Properties and Their Applications as a Soil Ameliorant. In Amelioration Technology for Soil Sustainability; Rathoure, A.K., Ed.; IGI Global: Hershey, PA, USA, 2019; pp. 59–89. [Google Scholar] [CrossRef] [Green Version]
- Pacewska, B.; Wilińska, I. Usage of supplementary cementitious materials: Advantages and limitations. J. Therm. Anal. Calorim. 2020, 142, 371–393. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Stoica, A.E.; Vrabec, M.; Smuc Rogan, N.; Sturm, S.; Ow-Yang, C.; Gulgun, M.A.; Bundur, Z.B.; Ciuca, I.; Vasile, B.S. End-of-Life Materials Used as Supplementary Cementitious Materials in the Concrete Industry. Materials 2020, 13, 1954. [Google Scholar] [CrossRef] [Green Version]
- Fuller, A.; Maier, J.; Karampinis, E.; Kalivodova, J.; Grammelis, P.; Kakaras, E.; Scheffknecht, G. Fly Ash Formation and Characteristics from (co-)Combustion of an Herbaceous Biomass and a Greek Lignite (Low-Rank Coal) in a Pulverized Fuel Pilot-Scale Test Facility. Energies 2018, 11, 1581. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, A.; Priyadarshini, S.; Acharath Mohanakrishnan, A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Yadav, V.K.; Saxena, P.; Lal, C.; Gnanamoorthy, G.; Choudhary, N.; Singh, B.; Tavker, N.; Kalasariya, H.; Kumar, P. Synthesis and Characterization of Mullites From Silicoaluminous Fly Ash Waste. Int. J. Appl. Nanotechnol. Res. (IJANR) 2021, 5, 18. [Google Scholar] [CrossRef]
- Behera, A.; Mohapatra, S. Challenges in Recovery of Valuable and Hazardous Elements from Bulk Fly Ash and Options for Increasing Fly Ash Utilization. In Coal Fly Ash Beneficiation-Treatment of Acid Mine Drainage with Coal Fly Ash; Gitari, S.A.A.a.M.W., Ed.; Intechopen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Chand Malav, L.; Yadav, K.K.; Gupta, N.; Kumar, S.; Sharma, G.K.; Krishnan, S.; Rezania, S.; Kamyab, H.; Pham, Q.B.; Yadav, S.; et al. A review on municipal solid waste as a renewable source for waste-to-energy project in India: Current practices, challenges, and future opportunities. J. Clean. Prod. 2020, 277, 123227. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. The current scenario of thermal power plants and fly ash: Production and utilization with a focus in India. Int. J. Adv. Eng. Res. Dev. 2018, 5, 768–777. [Google Scholar]
- Tarafdar, A.; Sinha, A. Polycyclic Aromatic Hydrocarbons (PAHs) Pollution Generated from Coal-Fired Thermal Power Plants: Formation Mechanism, Characterization, and Profiling: Characterization and Control. In Pollutants from Energy Sources, Energy, Environment, and Sustainability; Agarwal, R.A.A., Gupta, T., Sharma, N., Eds.; Springer: Singapore, 2019; pp. 73–90. [Google Scholar] [CrossRef]
- Cabral-Pinto, M.M.S.; Inácio, M.; Neves, O.; Almeida, A.A.; Pinto, E.; Oliveiros, B.; Ferreira da Silva, E.A. Human Health Risk Assessment Due to Agricultural Activities and Crop Consumption in the Surroundings of an Industrial Area. Exp. Health 2020, 12, 629–640. [Google Scholar] [CrossRef]
- Hower, J.; Groppo, J.; Graham, U.; Ward, C.; Kostova, I.; Maroto-Valer, M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.S.; Sarker, P.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavker, N.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Removal of Cadmium and Chromium by Mixture of Silver Nanoparticles and Nano-Fibrillated Cellulose Isolated from Waste Peels of Citrus Sinensis. Polymers 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Aqel, A.; El-Nour, K.M.M.A.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Jovic, D.; Jacevic, V.; Kuca, K.; Borisev, I.; Mrdjanovic, J.; Petrovic, D.; Seke, M.; Djordjevic, A. The Puzzling Potential of Carbon Nanomaterials: General Properties, Application, and Toxicity. Nanomaterials 2020, 10, 1508. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis of electrospun polyacrylonitrile-derived carbon fibers and comparison of properties with bulk form. PLoS ONE 2018, 13, e0201345. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, J.; Yang, P.; Qiao, X.; Tian, F. Polycyclic aromatic hydrocarbons in Dalian soils: Distribution and toxicity assessment. J. Environ. Monit. 9, 199–204. [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 24. [Google Scholar] [CrossRef]
- Moon, M.-W.; Kim, H.-Y.; Wang, A.; Vaziri, A. Nanostructured Carbon Materials. J. Nanomater. 2015, 2015, 916834. [Google Scholar] [CrossRef]
- Wildgoose, G.; Banks, C.; Compton, R. Metal Nanoparticles and Related Materials Supported on Carbon Nanotubes: Methods and Applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Fetzer, J. The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons. Polycycl. Aromat. Compd. (New York: Wiley) 2000, 27, 143–147. [Google Scholar] [CrossRef]
- Liu, G.; Niu, Z.; Van Niekerk, D.; Xue, J.; Zheng, L. Polycyclic Aromatic Hydrocarbons (PAHs) from Coal Combustion: Emissions, Analysis, and Toxicology. Rev. Environ. Contam. Toxicol. 2008, 192, 1–28. [Google Scholar] [CrossRef]
- Liu, D.; Duan, Y.Y.; Yang, Z.; Yu, H.T. A new route for unburned carbon concentration measurements eliminating mineral content and coal rank effects. Sci. Rep. 2014, 4, 4567. [Google Scholar] [CrossRef] [Green Version]
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar] [CrossRef] [Green Version]
- Salah, N.; Al-Ghamdi, A.; Memic, A.; Habib, S.; Khan, Z. Formation of Carbon Nanotubes from Carbon Rich Fly Ash: Growth Parameters and Mechanism. Mater. Manuf. Process. 2015, 31, 150811005209005. [Google Scholar] [CrossRef]
- Hsieh, Y.-M.; Tsai, M.-S. Physical and chemical analyses of unburned carbon from oil-fired fly ash. Carbon 2003, 41, 2317–2324. [Google Scholar] [CrossRef]
- Algarni, A.; Salah, N.; Bourchak, M.; Jilani, A.; Alshahrie, A.; Nahas, M.N. Polymer composite reinforced with nanoparticles produced from graphitic carbon-rich fly ash. J. Compos. Mater. 2016, 51, 2675–2685. [Google Scholar] [CrossRef]
- Mofarrah, A.; Husain, T. Use of Heavy Oil Fly Ash as a Color Ingredient in Cement Mortar. Int. J. Concr. Struct. Mater. 2013, 7, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.; Kumar, S.; Ratha, D. Physiochemical and leaching characteristics of fly and bottom ash. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2377–2382. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2017; IEA: France, Paris, 2017. [Google Scholar]
- Miricioiu, M.G.; Niculescu, V.C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoňová, L. Unburned carbon from coal combustion ash: An overview. Fuel Process. Technol. 2015, 134, 136–158. [Google Scholar] [CrossRef]
- Wilcox, J.; Wang, B.; Rupp, E.; Taggart, R.; Hsu-Kim, H.; Oliveira, M.; Cutruneo, C.; Taffarel, S.; Silva, L.; Hopps, S.; et al. Observations and Assessment of Fly Ashes from High-Sulfur Bituminous Coals and Blends of High-Sulfur Bituminous and Subbituminous Coals: Environmental Processes Recorded at the Macro- and Nanometer Scale. Energy Fuels 2015, 29, 7168–7177. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal Biomass Conversion: A Review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Saptoro, A.; Tade, M.O. Prediction and Monitoring of Unburnt Carbon in Fly Ash in Coal-Fired Power Plant; Curtin University of Technology: Perth, Australia, 2006. [Google Scholar]
- Boycheva, S.; Zgureva, D.; Lazarova, K.; Babeva, T.; Popov, C.; Lazarova, H.; Popova, M. Progress in the Utilization of Coal Fly Ash by Conversion to Zeolites with Green Energy Applications. Materials 2020, 13, 2014. [Google Scholar] [CrossRef]
- Sequeira, M.D.; Castilho, A.M.; Dinis, P.A.; Tavares, A.O. Impact Assessment and Geochemical Background Analysis of Surface Water Quality of Catchments Affected by the 2017 Portugal Wildfires. Water 2020, 12, 2742. [Google Scholar] [CrossRef]
- Pinto, M.M.S.C.; Silva, E.A.F.d.; Silva, M.M.V.G.; Melo-Gonçalves, P.; Candeias, C. Environmental Risk Assessment Based on High-Resolution Spatial Maps of Potentially Toxic Elements Sampled on Stream Sediments of Santiago, Cape Verde. Geosciences 2014, 4, 297–315. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Liu, G.; Niu, Z.; Chou, C.-L.; Qi, C.; Zheng, L.; Zhang, H. Factors That Influence the Extraction of Polycyclic Aromatic Hydrocarbons from Coal. Energy Fuels 2007, 21, 881–890. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G.; Zhang, J.; Chou, C.L.; Liu, J. Abundances of polycyclic aromatic hydrocarbons (PAHs) in 14 chinese and american coals and their relation to coal rank and weathering. Energy Fuels 2010, 24, 6061–6066. [Google Scholar] [CrossRef]
- Wei, X.-Y.; Gui, J.; Wang, Y.; Li, P.; Zong, Z.-M. Characterization of Biomarkers and Structural Features of Condensed Aromatics in Xianfeng Lignite. Energy Fuels 2013, 27, 7369–7378. [Google Scholar] [CrossRef]
- Bowman, D.T.; Jobst, K.J.; Helm, P.A.; Kleywegt, S.; Diamond, M.L. Characterization of Polycyclic Aromatic Compounds in Commercial Pavement Sealcoat Products for Enhanced Source Apportionment. Environ. Sci. Technol. 2019, 53, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Achten, C.; Hofmann, T. Native polycyclic aromatic hydrocarbons (PAH) in coals-A hardly recognized source of environmental contamination. Sci. Total Environ. 2009, 407, 2461–2473. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Q.; Zhao, N.; Jin, B.; Zhuang, X.; Bai, Z. Distributions and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils around a Chemical Plant in Shanxi, China. Int. J. Environ. Res Public Health 2017, 14, 1198. [Google Scholar] [CrossRef]
- Gerardo, B.; Cabral Pinto, M.; Nogueira, J.; Pinto, P.; Almeida, A.; Pinto, E.; Reis, A.; Diniz, L.; Moreira, P.; Simões, M.; et al. Associations between Trace Elements and Cognitive Decline: An Exploratory 5-Year Follow-Up Study of an Elderly Cohort. Int. J. Environ. Res Public Health 2020, 17, 6051. [Google Scholar] [CrossRef]
- Liu, K.; Heltsley, R.; Zou, D.; Pan, W.-P.; Riley, J.T. Polyaromatic Hydrocarbon Emissions in Fly Ashes from an Atmospheric Fluidized Bed Combustor Using Thermal Extraction Coupled with GC/TOF-MS. Energy Fuels 2002, 16, 330–337. [Google Scholar] [CrossRef]
- Alterary, S.; Marei, N.H. The Impact of Coal Fly Ash Purification on Its Antibacterial Activity. Minerals 2020, 10, 1002. [Google Scholar] [CrossRef]
- Chen, H.-J.; Shih, N.-H.; Wu, C.-H.; Lin, S.-K. Effects of the Loss on Ignition of Fly Ash on the Properties of High-Volume Fly Ash Concrete. Sustainability 2019, 11, 2704. [Google Scholar] [CrossRef] [Green Version]
- Golewski, G.L. Energy Savings Associated with the Use of Fly Ash and Nanoadditives in the Cement Composition. Energies 2020, 13, 2184. [Google Scholar] [CrossRef]
- Fan, M.; Brown, R. Comparison of the Loss-on-Ignition and Thermogravimetric Analysis Techniques in Measuring Unburned Carbon in Coal Fly Ash. Fuel Energy Abstr. 2001, 43. [Google Scholar] [CrossRef]
- Mohebbi, M.; Rajabipour, F.; Scheetz, B.E. Evaluation of Two-Atmosphere Thermogravimetric Analysis for Determining the Unburned Carbon Content in Fly Ash. Adv. Civ. Eng. Mater. 2017, 6, 258–279. [Google Scholar] [CrossRef]
- Bartoéová, L.; Juchelková, D.; Klika, Z. On Unburned Carbon in Coal Ash from Various Combustion Units. Int. J. Mater. Metall. Eng. 2011, 5, 280–283. [Google Scholar]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Mikhailova, A.; Goldberg, M.; Kondratiev, A. Complex utilisation of ekibastuz brown coal fly ash: Iron & carbon separation and aluminum extraction. J. Clean. Prod. 2019, 218, 192–201. [Google Scholar] [CrossRef]
- Dinis, P.A.; Garzanti, E.; Hahn, A.; Vermeesch, P.; Cabral-Pinto, M. Weathering indices as climate proxies. A step forward based on Congo and SW African river muds. Earth Sci. Rev. 2020, 201. [Google Scholar] [CrossRef]
- Lasagni, M.; Collina, E.; Ferri, M.; Tettamanti, M.; Pitea, D. Total Organic Carbon in Fly Ash from MSW Incinerators as a Potential Combustion Indicator: Setting Up of the Measurement Methodology and Preliminary Evaluation. Waste Manag. Res. 1997, 15, 507–521. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Wei, G.; Liu, H.; Liu, F.; Zeng, T.; Liu, G.; Zhou, J. Experimental Investigation of the Decarburization Behavior of Medical Waste Incinerator Fly Ash (MWIFA). Processes 2018, 6, 186. [Google Scholar] [CrossRef] [Green Version]
- Eisele, T.; Kawatra, S. Use of froth flotation to remove unburned carbon from fly ash. Miner. Process. Extr. Metall. Rev. 2002, 23, 1–10. [Google Scholar] [CrossRef]
- Wrona, J.; Żukowski, W.; Bradło, D.; Czupryński, P. Recovery of Cenospheres and Fine Fraction from Coal Fly Ash by a Novel Dry Separation Method. Energies 2020, 13, 3576. [Google Scholar] [CrossRef]
- Fraunholcz, N. Separation of waste plastics by froth flotation—A review, part I. Miner. Eng. 2004, 17, 261–268. [Google Scholar] [CrossRef]
- Bournival, G.; Ata, S.; Jameson, G. Bubble and Froth Stabilizing Agents in Froth Flotation. Miner. Process. Extr. Metall. Rev. 2017, 38. [Google Scholar] [CrossRef]
- Liaoa, Y.; Caoa, Y.; Hub, Z.; Taoc, X. A new preparation scheme for a difficult-to-float coking coal by column flotation following grinding. J. South. Afr. Inst. Min. Metall. 2015, 115, 161–164. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Varnavskaya, A.; Ju, D. Magnetite and Carbon Extraction from Coal Fly Ash Using Magnetic Separation and Flotation Methods. Minerals 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Uçurum, M.; Toraman, Ö.Y.; Depci, T.; Yoğurtçuoğlu, E. A Study on Characterization and Use of Flotation to Separate Unburned Carbon in Bottom Ash from Çayirhan Power Plant. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 562–574. [Google Scholar] [CrossRef]
- Das, A.; Sarkar, B.; Ari, V.; Roy, S. Efficient Recovery of Combustibles from Coking Coal Fines. Miner. Process. Extr. Metall. Rev. 2010, 31, 236–249. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Zheng, N.; Sun, Z. Experimental investigation of the attachment of unburned carbon in coal fly ash to a stationary air bubble in aqueous solutions. Fuel 2021, 285, 119080. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, W.; Hu, S.; Yang, Z.; Liu, X.; Fan, Z. Comprehensive utilization of foundry dust: Coal powder and clay minerals separation by ultrasonic-assisted flotation. J Hazard Mater 2021, 402, 124124. [Google Scholar] [CrossRef]
- Buha, J.; Mueller, N.; Nowack, B.; Ulrich, A.; Losert, S.; Wang, J. Physical and Chemical Characterization of Fly Ashes from Swiss Waste Incineration Plants and Determination of the Ash Fraction in the Nanometer Range. Environ. Sci. Technol. 2014, 48, 4765–4773. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, F.; Xu, M.; Gui, X.; Li, H.; Li, G.; Xia, Y.; Han, H. Separation of unburned carbon from coal fly ash: A review. Powder Technol. 2019, 353, 372–384. [Google Scholar] [CrossRef]
- Rubio, B.; Izquierdo, M.T.; Mayoral, M.; Bona, M.T.; Martínez-Tarazona, M. Preparation and Characterisation of Carbon-Enriched Coal Fly Ash. J. Environ. Manag. 2008, 88, 1562–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, M.L.; Champagne, K.J.; Soong, Y.; Finseth, D.H. Parametric study of the column oil agglomeration of fly ash. Fuel 2001, 80, 867–871. [Google Scholar] [CrossRef]
- Baltrus, J.P.; Wells, A.W.; Fauth, D.J.; Diehl, J.R.; White, C.M. Characterization of Carbon Concentrates from Coal-Combustion Fly Ash. Energy Fuels 2001, 15, 455–462. [Google Scholar] [CrossRef]
- Nguyen, H.G.T.; Horn, J.C.; Bleakney, M.; Siderius, D.W.; Espinal, L. Understanding Material Characteristics through Signature Traits from Helium Pycnometry. Langmuir 2019, 35, 2115–2122. [Google Scholar] [CrossRef]
- Bartoňová, L.; Čech, B.; Ruppenthalová, L.; Majvelderova, V.; Juchelková, D.; Klika, Z. Effect of unburned carbon content in fly ash on the retention of 12 elements out of coal-combustion flue gas. J. Environ. Sci. 2012, 24, 1624–1629. [Google Scholar] [CrossRef]
- Li, G.; Deng, L.; Liu, J.; Cao, Y.; Zhang, H.; Ran, J. A New Technique for Removing Unburned Carbon From Coal Fly Ash at an Industrial Scale. Int. J. Coal Prep. Util. 2015, 35, 150527094541002. [Google Scholar] [CrossRef]
- Gray, M.; Champagne, K.; Soong, Y.; Killmeyer, R.; Maroto-Valer, M.; Andresen, J.; Ciocco, M.; Zandhuis, P. Physical Cleaning of High Carbon Fly Ash. Fuel Process. Technol. 2002, 76, 11–21. [Google Scholar] [CrossRef]
- Kim, J.-K.; Cho, H.-C.; Kim, S.-C. Removal of unburned carbon from coal fly ash using a pneumatic triboelectrostatic separator. J. Environ. Sci. Health Part A 2001, 36, 1709–1724. [Google Scholar] [CrossRef]
- Wierzchowski, K.; Białecka, B.; Moszko, J.; Klupa, A. Characterization of unburned carbon separated from power plant slag. Int. J. Environ. Sci. Technol. 2020, 17, 2499–2510. [Google Scholar] [CrossRef] [Green Version]
- Cabral Pinto, M.M.S.; Ferreira da Silva, E.A. Heavy Metals of Santiago Island (Cape Verde) Alluvial Deposits: Baseline Value Maps and Human Health Risk Assessment. Int. J. Environ. Res. Public Health 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groppo, J.; Brooks, S.M. Froth flotation method for removing ultrafine carbon from fly ashes. Fuel Energy Abstr. 1996, 37, 186. [Google Scholar] [CrossRef]
- Butcher, D.; Rowson, N. Electrostatic Separation of Pyrite from Coal. Magn. Electr. Sep. 1995, 6. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Soong, Y.; Schoffstall, M.R.; Gray, M.; Knoer, J.P.; Champagne, K.J.; Jones, R.J.; Fauth, D. Dry beneficiation of high loss-on-ignition fly ash. Sep. Purif. Technol. 2002, 26, 177–184. [Google Scholar] [CrossRef]
- Cangialosi, F.; Notarnicola, M.; Liberti, L.; Stencel, J. The role of weathering on fly ash charge distribution during triboelectrostatic beneficiation. J. Hazard. Mater. 2008, 164, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Mohapatra, S.K.; Singh, R.I. Review on CFD Modelling of Fluidized Bed Combustion Systems based on Biomass and Co-firing. J. Inst. Eng. (India) Ser. C 2018, 99, 449–474. [Google Scholar] [CrossRef]
- Zacharia, R.; Rather, S.U. Review of Solid State Hydrogen Storage Methods Adopting Different Kinds of Novel Materials. J. Nanomater. 2015, 2015, 914845. [Google Scholar] [CrossRef] [Green Version]
- Ban, H.; Li, T.; Schaefer, J.L.; Stencel, J. Triboelectrostatic separation of unburned carbon from fly ash. Prepr. Pap. Am. Chem. Soc. Div. Fuel Chem. 1996, 41. [Google Scholar]
- Maroto-Valer, M.; Zhang, Y.; Granite, E.; Tang, Z.; Pennline, H. Effect of porous structure and surface functionality on the mercury capacity of a fly ash carbon and its activated sample. Fuel 2005, 84, 105–108. [Google Scholar] [CrossRef]
- Walker, A.; Wheelock, T. Separation of Carbon from Fly Ash Using Froth Flotation. Coal Prep. 2006, 26, 235–250. [Google Scholar] [CrossRef]
- Hurt, R.; Davis, K.; Yang, N.; Headley, T.; Mitchell, G. Residual carbon from pulverized-coal-fired boilers. 2. Morphology and physicochemical properties. Fuel 1995, 74, 1297–1306. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Li, P.; Jiajian, G.; Gu, F.; Su, F. Enhanced fluidized bed methanation over a Ni/Al2O3 catalyst for production of synthetic natural gas. Chem. Eng. J. 2013, 219, 183–189. [Google Scholar] [CrossRef]
- Barnard, A.; Russo, S.; Snook, I. Coexistence of bucky diamond with nanodiamond and fullerene carbon phases. Phys. Rev. B 2003, 68. [Google Scholar] [CrossRef]
- Francis, A.H. Electronic Structure Calculations on Fullerenes and Their Derivatives by Jerzy Cioslowski (Florida State University). Oxford University Press: New York. 1995. ix + 281 pp. $65.00. ISBN 0-19-508806-9. J. Am. Chem. Soc. 1996, 118, 9458. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Dosodia, A.; Lal, C.; Singh, B.P.; Mathur, R.B.; Sharma, D. Development of Catalyst Free Carbon Nanotubes from Coal and Waste Plastics. Fuller. Nanotub. Carbon Nanostructures 2009, 17, 567–582. [Google Scholar] [CrossRef]
- Gieré, R.; Blackford, M.; Smith, K. TEM Study of PM 2.5 Emitted from Coal and Tire Combustion in a Thermal Power Station. Environ. Sci. Technol. 2006, 40, 6235–6240. [Google Scholar] [CrossRef]
- Tiwari, A.J.; Ashraf-Khorassani, M.; Marr, L.C. C60 fullerenes from combustion of common fuels. Sci. Total Environ. 2016, 547, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Saikia, J.; Khare, P.; Saikia, P.; Saikia, B.K. Polycyclic aromatic hydrocarbons (PAHs) around tea processing industries using high-sulfur coals. Environ. Geochem. Health 2017, 39, 1101–1116. [Google Scholar] [CrossRef]
- Martinello, K.; Hower, J.C.; Dotto, G.L.; Ramos, C.G.; Schnorr, C.E.; Pinto, D. Deposition of nanoparticles on school eyeglasses in urban and rural areas: A methodology for a more real assessment of the possible impacts. Geosci. Front. 2021. [Google Scholar] [CrossRef]
- Konchits, A.A.; Shanina, B.D.; Krasnovyd, S.V.; Burya, A.I.; Kuznetsova, O.Y. Paramagnetic Properties of Fullerene-Derived Nanomaterials and Their Polymer Composites: Drastic Pumping Out Effect. Nanoscale Res. Lett. 2017, 12, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hower, J.C.; Graham, U.M.; Dozier, A.; Tseng, M.T.; Khatri, R.A. Association of the Sites of Heavy Metals with Nanoscale Carbon in a Kentucky Electrostatic Precipitator Fly Ash. Environ. Sci. Technol. 2008, 42, 8471–8477. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.T.; Satpathy, S.K.; Manna, I.; Chakraborty, K.K.; Nando, G.B. Preparation and Characterization of Nano structured Materials from Fly Ash: A Waste from Thermal Power Stations, by High Energy Ball Milling. Nanoscale Res. Lett. 2007, 2, 397. [Google Scholar] [CrossRef] [Green Version]
- Monthioux, M.; Kuznetsov, V. Who should be given the credit for the discovery of carbon nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martínez-Hernández, A.; Cosultchi, A.; Rodríguez Talavera, J.R.; Castaño, V. Naturally produced carbon nanotubes. Chem. Phys. Lett. 2003, 373, 272–276. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Wabaidur, S.M. Application of carbon nanotubes in extraction and chromatographic analysis: A review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Alsharef, J.; Taha, M.; Khan, T. Physical dispersion of nanocarbons in composites—A review. J. Teknol. 2017, 79, 68–69. [Google Scholar] [CrossRef] [Green Version]
- Batakliev, T.; Petrova-Doycheva, I.; Angelov, V.; Georgiev, V.; Ivanov, E.; Kotsilkova, R.; Casa, M.; Cirillo, C.; Adami, R.; Sarno, M.; et al. Effects of Graphene Nanoplatelets and Multiwall Carbon Nanotubes on the Structure and Mechanical Properties of Poly(lactic acid) Composites: A Comparative Study. Appl. Sci. 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Hatui, G.; Bhattacharya, P.; Sahoo, S.; Dhibar, S.; Das, C.K. Combined effect of expanded graphite and multiwall carbon nanotubes on the thermo mechanical, morphological as well as electrical conductivity of in situ bulk polymerized polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2014, 56, 181–191. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, s019–s11671. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Saikia, B.K.; Baruah, B.P. Formation of carbon nano-balls and carbon nano-tubes from northeast Indian Tertiary coal: Value added products from low grade coal. Gondwana Res. 2016, 31, 295–304. [Google Scholar] [CrossRef]
- Oliveira, M.; Marostega, F.; Taffarel, S.; Saikia, B.K.; Waanders, F.; Martinello, K.; Baruah, B.P.; Silva, L. Nano-mineralogical investigation of coal and fly ashes from coal-based captive power plant (India): An introduction of occupational health hazards. Sci. Total Environ. 2013, 468–469C, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Hintsho, N.; Shaikjee, A.; Masenda, H.; Naidoo, D.; Billing, D.; Franklyn, P.; Durbach, S. Direct synthesis of carbon nanofibers from South African coal fly ash. Nanoscale Res. Lett. 2014, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Kronbauer, M.; Izquierdo, M.; Dai, S.; Waanders, F.; Wagner, N.; Mastalerz, M.; Hower, J.; Oliveira, M.; Taffarel, S.; Bizani, D.; et al. Geochemistry of ultra-fine and nano-compounds in coal gasification ashes: A synoptic view. Sci. Total Environ. 2013, 456–457C, 95–103. [Google Scholar] [CrossRef]
- Martinello, K.; Oliveira, M.; Molossi, F.; Ramos, C.; Calesso Teixeira, E.; Kautzmann, R.; Silva, L. Direct identification of hazardous elements in ultra-fine and nanominerals from coal fly ash produced during diesel co-firing. Sci. Total Environ. 2013, 470–471C, 444–452. [Google Scholar] [CrossRef]
- Silva, L.; Martinello, K.; Mardon, S.; Hower, J.; Serra-Rodríguez, C. Fullerenes and Metallofullerenes in Coal-Fired Stoker Fly Ash. Coal Combust. Gasif. Prod. 2010, 2. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karim Khanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, S.; Moazzenchi, B. Carbon nanotube and its applications in textile industry—A review. J. Text. Inst. 2018, 109, 1653–1666. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications. Polymers (Basel) 2019, 11, 1667. [Google Scholar] [CrossRef] [Green Version]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Coetzee, D.; Venkataraman, M.; Militky, J.; Petru, M. Influence of Nanoparticles on Thermal and Electrical Conductivity of Composites. Polymers (Basel) 2020, 12, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakakis, G.; Tomara, G.; Datsyuk, V.; Sygellou, L.; Bakolas, A.; Tasis, D.; Parthenios, J.; Krontiras, C.; Georga, S.; Galiotis, C.; et al. Mechanical, Electrical, and Thermal Properties of Carbon Nanotube Buckypapers/Epoxy Nanocomposites Produced by Oxidized and Epoxidized Nanotubes. Materials 2020, 13, 4308. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wang, Y.; Ren, Z. Physics and applications of aligned carbon nanotubes. Adv. Phys. 2011, 60, 553–678. [Google Scholar] [CrossRef]
- Camilli, L.; Passacantando, M. Advances on Sensors Based on Carbon Nanotubes. Chemosensors 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Coal fly ash an amendment to container substrate for Spathiphyllum production. Bioresour. Technol. 2006, 97, 1920–1926. [Google Scholar] [CrossRef]
- Chen, Y.; Shah, N.; Huggins, F.E.; Huffman, G.P. Transmission Electron Microscopy Investigation of Ultrafine Coal Fly Ash Particles. Environ. Sci. Technol. 2005, 39, 1144–1151. [Google Scholar] [CrossRef]
- Murr, L.; Soto, K. A TEM study of soot, carbon nanotubes, and related fullerene nanopolyhedra in common fuel-gas combustion sources. Mater. Charact. 2005, 554, 50–65. [Google Scholar] [CrossRef]
- Lou, L.; Luo, L.; Wang, W.; Xu, X.; Hou, J.; Xun, B.; Chen, Y. Impact of black carbon originated from fly ash and soot on the toxicity of pentachlorophenol in sediment. J. Hazard. Mater. 2011, 190, 474–479. [Google Scholar] [CrossRef]
- Veranth, J.M.; Fletcher, T.H.; Pershing, D.W.; Sarofim, A.F. Measurement of soot and char in pulverized coal fly ash. Fuel 2000, 79, 1067–1075. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Evans, S.; Ge, Y.; Zhu, Z.; Tu, Y.; Yang, X. Confined Assembly of Hollow Carbon Spheres in Carbonaceous Nanotube: A Spheres-in-Tube Carbon Nanostructure with Hierarchical Porosity for High-Performance Supercapacitor. Small 2018, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shah, N.; Huggins, F.E.; Huffman, G.P. Investigation of the Microcharacteristics of PM2.5 in Residual Oil Fly Ash by Analytical Transmission Electron Microscopy. Environ. Sci. Technol. 2004, 38, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, R.; Sader, K.; Warner, J.; Plant, S.; Porfyrakis, K.; Nellist, P.; Briggs, G.; Cockayne, D. Direct Imaging and Chemical Identification of the Encapsulated Metal Atoms in Bimetallic Endofullerene Peapods. ACS Nano 2010, 4, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Gunture; Kaushik, J.; Garg, A.K.; Saini, D.; Khare, P.; Sonkar, S.K. Pollutant Diesel Soot Derived Onion-like Nanocarbons for the Adsorption of Organic Dyes and Environmental Assessment of Treated Wastewater. Ind. Eng. Chem. Res. 2020, 59, 12065–12074. [Google Scholar] [CrossRef]
- Graham, U.; Dozier, A.; Khatri, R.; Tseng, M.; Hower, J.; Davis, B. Ultra-Fine PM Derived from Fullerene-Like Carbon in Electrostatic Precipitator Fly Ash. In Proceedings of the 2008 AIChE Annual Meeting, Philadelphia, PA, USA, 16–21 November 2008. [Google Scholar]
- Everson, R.; Neomagus, H.; Kasaini, H.; Njapha, D. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam. Fuel 2006, 85, 1076–1082. [Google Scholar] [CrossRef]
- Pedersen, K.H.; Jensen, A.; Skjøth-Rasmussen, M.; Dam-Johansen, K. A review of the interference of carbon containing fly ash with air entrainment in concrete. Prog. Energy Combust. Sci. 2008, 34, 135–154. [Google Scholar] [CrossRef]
- Bralewska, K.; Rakowska, J. Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. Int. J. Environ. Res. Public Health 2020, 17, 3202. [Google Scholar] [CrossRef]
- Ruwei, W.; Jiamei, Z.; Jingjing, L.; Liu, G. Levels and Patterns of Polycyclic Aromatic Hydrocarbons in Coal-Fired Power Plant Bottom Ash and Fly Ash from Huainan, China. Arch. Environ. Contam. Toxicol. 2013, 65, 193–202. [Google Scholar] [CrossRef]
- Lima, A.; Farrington, J.; Reddy, C. Combustion-Derived Polycyclic Aromatic Hydrocarbons in the Environment—A Review. Environ. Forensics 2005, 6, 109–131. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Romero, R.; Sharma, K.; Singh, J. Applications of Nanoparticles in Wastewater Treatment. In Nanobiotechnology in Bioformulations; Springer: Cham, Switzerland, 2019; pp. 395–418. [Google Scholar] [CrossRef]
- Reizer, E.; Csizmadia, I.G.; Palotás, Á.B.; Viskolcz, B.; Fiser, B. Formation Mechanism of Benzo(a)pyrene: One of the Most Carcinogenic Polycyclic Aromatic Hydrocarbons (PAH). Molecules 2019, 24, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirondart, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Bily, A.; Chemat, F. Comparison between Pressurized Liquid Extraction and Conventional Soxhlet Extraction for Rosemary Antioxidants, Yield, Composition, and Environmental Footprint. Foods 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Koch, B.; Lutermann, C.; Dott, W. Efficiency of Different Methods and Solvents for the Extraction of Polycyclic Aromatic Hydrocarbons from Soils. Int. J. Environ. Anal. Chem. 2003, 83, 21–32. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical Methods for Polycyclic Aromatic Hydrocarbons and their Global Trend of Distribution in Water and Sediment: A Review. In Recent Insights in Petroleum Science and Engineering; Intech: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Akila, S.; Srinivasan, P. Production of Polyphenol from Phyllanthus Emblica using Soxhlet Extraction Process. Int. J. Recent Technol. Eng. 2019, 8, 5010–5012. [Google Scholar] [CrossRef]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Haneef, T.; Mustafa, M.R.U.; Wan Yusof, K.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Yang, L.; Wu, J.; Zheng, M.; Cao, Z.; Li, C.; Shi, M.; Liu, G. Non-target screening of organic pollutants and target analysis of halogenated polycyclic aromatic hydrocarbons in the atmosphere around metallurgical plants by high-resolution GC/Q-TOF-MS. Environ. Sci. Eur. 2020, 32, 96. [Google Scholar] [CrossRef]
- Mastral, A.; Garcia, T.; Callén, M.; Lopez, J.; Murillo, R.; Navarro, M. Effects of Limestone on Polycyclic Aromatic Hydrocarbon Emissions during Coal Atmospheric Fluidized Bed Combustion. Energy Fuels 2001, 15, 1469–1474. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [Green Version]
- Clack, H.L. Estimates of Increased Black Carbon Emissions from Electrostatic Precipitators during Powdered Activated Carbon Injection for Mercury Emissions Control. Environ. Sci. Technol. 2012, 46, 7327–7333. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandeep; Shukla, P. Microbial Nanotechnology for Bioremediation of Industrial Wastewater. Front Microbiol 2020, 11, 590631. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Chen, Y.; Frackowiak, E.; Holzinger, M.; Koratkar, N.; Meunier, V.; Mikhailovsky, S.; Strano, M.; Tascon, J.; Terrones, M. Carbon Science Perspective in 2020: Current Research and Future Challenges. Carbon 2020, 161, 373–391. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, W. Low temperature selective catalytic reduction of nitric oxide with an activated carbon-supported zero-valent iron catalyst. Rsc Adv. 2020, 10, 42613–42618. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Depero, L.E.; Bontempi, E. Review of the Reuse Possibilities Concerning Ash Residues from Thermal Process in a Medium-Sized Urban System in Northern Italy. Sustainability 2020, 12, 4193. [Google Scholar] [CrossRef]
- Badenhorst, C.; Santos, C.; Lázaro-Martínez, J.; Białecka, B.; Cruceru, M.; Guedes, A.; Guimarães, R.; Moreira, K.; Predeanu, G.; Suárez-Ruíz, I.; et al. Assessment of Graphitized Coal Ash Char Concentrates as a Potential Synthetic Graphite Source. Minerals 2020, 10, 986. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.; Choi, N. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef] [Green Version]
- Pransisco, P.; Singh, M.S.; Shuaib, M.; Shukur, M.F.; Joseph, E. 3D graphene/fly ash waste material for hybrid supercapacitor electrode: Specific capacitance analysis. Mater. Und Werkst. 2020, 51, 713–718. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.T.; Ahmad, A.L.; Zein, S.H.S.; Tan, S.H. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz. J. Chem. Eng. 2010, 27, 227–242. [Google Scholar] [CrossRef]





| Descriptions | 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash production | 131.09 | 145.42 | 163.56 | 172.87 | 184.14 | 176.74 | 169.25 | 196.44 | 217.04 | 226.13 |
| Fly ash utilization | 73.13 | 85.05 | 100.37 | 99.62 | 102.54 | 107.77 | 107.10 | 131.87 | 168.40 | 187.81 |
| % Utilization | 55.79 | 58.48 | 61.37 | 57.37 | 55.69 | 60.97 | 63.28 | 67.13 | 77.59 | 83.05 |
| Method | References | Efficiency/Findings |
|---|---|---|
| 1. Wet Separation Method | ||
| Froth flotation | [64,78,96] | Simple method; no liquid media used; and less space for machinery, less energy required, and high recovering capacity |
| Oil agglomeration | [77,78] | Provides highly pure unburned carbon with higher recoveries |
| Density separation | [79,97] | Provides unburnt high purity carbon; no use of chemicals; and no contamination or alterations in the properties of carbon |
| 2. Dry Separation Method | ||
| Separation by size classification | [87] | Separates the UC particles on the basis of size and density |
| Electrostatic separation | [78,90,91] | Separates the UC particles on the basis of electron affinity |
| Incipient fluidization | [98] | Provides highly pure carbon; No danger of leakage or contamination. |
| Tribo-electrostatic separation | [95] | Electrostatic-based separation of UC particles between two electrodes |
| Type of carbon NMs | References | Efficiency/Description of CNMs |
|---|---|---|
| Fullerene (C60) | [100,102,104,105] | Hollow, spherical |
| Nanocarbon and nanocoating | [108,109,140] | Nanoscale sooty or graphitic fullerene-like carbons; porous nanocoating |
| Carbon nanotubes | [31,102,110,120] | SWCNTs and MWCNTs; diameter of 8-20 nm; amorphous and crystalline nature |
| Carbon nanoballs | [132] | 5–10 nm |
| Carbon onions | [133] | Nanopolyhedra, onion-like, concentric |
| Chars | [24,141] | porous, carbon-rich particles |
| Soots | [108,136] | Ultrafine primary particles; aggregates of 10–50 nm diameter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, J.; Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.; Tavker, N.; Choudhary, N.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M.; Hamid, A.A. Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications. Crystals 2021, 11, 88. https://doi.org/10.3390/cryst11020088
Alam J, Yadav VK, Yadav KK, Cabral-Pinto MM, Tavker N, Choudhary N, Shukla AK, Ali FAA, Alhoshan M, Hamid AA. Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications. Crystals. 2021; 11(2):88. https://doi.org/10.3390/cryst11020088
Chicago/Turabian StyleAlam, Javed, Virendra Kumar Yadav, Krishna Kumar Yadav, Marina MS Cabral-Pinto, Neha Tavker, Nisha Choudhary, Arun Kumar Shukla, Fekri Abdulraqeb Ahmed Ali, Mansour Alhoshan, and Ali Awadh Hamid. 2021. "Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications" Crystals 11, no. 2: 88. https://doi.org/10.3390/cryst11020088
APA StyleAlam, J., Yadav, V. K., Yadav, K. K., Cabral-Pinto, M. M., Tavker, N., Choudhary, N., Shukla, A. K., Ali, F. A. A., Alhoshan, M., & Hamid, A. A. (2021). Recent Advances in Methods for the Recovery of Carbon Nanominerals and Polyaromatic Hydrocarbons from Coal Fly Ash and Their Emerging Applications. Crystals, 11(2), 88. https://doi.org/10.3390/cryst11020088