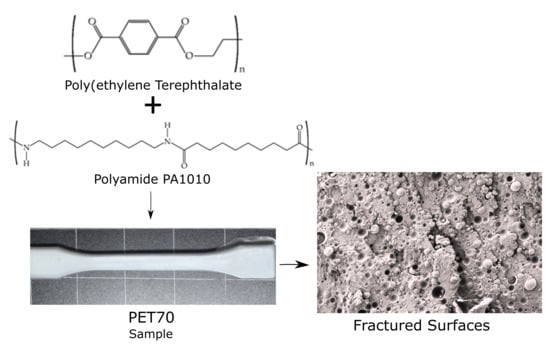
Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Manufacturing of Binary BioPET/BioPA Blends
2.3. Mechanical Characterization
2.4. Thermal Characterization
2.5. Morphology Characterization
2.6. Thermo-Mechanical Characterization
3. Results and Discussion
3.1. Mechanical Properties and Morphology of Binary BioPET/BioPA Blends
3.2. Thermal and Thermo-Mechanical Properties of Binary BioPET/BioPA Blends
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lahtela, V.; Hyvarinen, M.; Karki, T. Composition of Plastic Fractions in Waste Streams: Toward More Efficient Recycling and Utilization. Polymers 2019, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Spina, F.; Gentile, G.; Cocca, M.; Avella, M.; Carfagna, C.; Tealdo, G.; Errico, M.E. Recycling Polyethylene-Rich Plastic Waste from Landfill Reclamation: Toward an Enhanced Landfill-Mining Approach. Polymers 2019, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Chang. 2019, 9, 374. [Google Scholar] [CrossRef]
- Castilla-Cortazar, I.; Vidaurre, A.; Mari, B.; Campillo-Fernandez, A.J. Morphology, Crystallinity, and Molecular Weight of Poly(epsilon-caprolactone)/Graphene Oxide Hybrids. Polymers 2019, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucinska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Sato, H.; Ichikawa, Y.; Sunagawa, K.; Shigaki, Y. Development of an industrial production technology for high-molecular-weight polyglycolic acid. Polym. J. 2014, 46, 769–775. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly(butylene succinate) (PBS) and almond shell flour with different compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Khalil, F.; Galland, S.; Cottaz, A.; Joly, C.; Degraeve, P. Polybutylene succinate adipate/starch blends: A morphological study for the design of controlled release films. Carbohydr. Polym. 2014, 108, 272–280. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Lopez-Martinez, J.; Balart, R.; Stromberg, E.; Moriana, R. Reinforcing capability of cellulose nanocrystals obtained from pine cones in a biodegradable poly(3-hydroxybutyrate)/poly(epsilon-caprolactone) (PHB/PCL) thermoplastic blend. Eur. Polym. J. 2018, 104, 10–18. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly(lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr. Polym. 2016, 147, 60–68. [Google Scholar] [CrossRef]
- Domenek, S.; Feuilloley, P.; Gratraud, J.; Morel, M.H.; Guilbert, S. Biodegradability of wheat gluten based bioplastics. Chemosphere 2004, 54, 551–559. [Google Scholar] [CrossRef]
- Song, F.; Tang, D.-L.; Wang, X.-L.; Wang, Y.-Z. Biodegradable Soy Protein Isolate-Based Materials: A Review. Biomacromolecules 2011, 12, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, B.; Boronat, T.; Moriana, R.; Fenollar, O.; Balart, R. Green Composites Based on Wheat Gluten Matrix and Posidonia Oceanica Waste Fibers as Reinforcements. Polym. Compos. 2013, 34, 1663–1669. [Google Scholar] [CrossRef]
- Xue, Y.; Lofland, S.; Hu, X. Thermal Conductivity of Protein-Based Materials: A Review. Polymers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Yasin, T.; Hameed, A. Polyhydroxyalkanoates—What are the uses? Current challenges and perspectives. Crit. Rev. Biotechnol. 2015, 35, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, J.; Chen, G.-Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-Based (Bio)degradable Polymers as Environmentally Friendly Materials for Sustainable Development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef]
- Sharma, R.; Ray, A.R. Polyhydroxybutyrate, its copolymers and blends. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 1995, 35, 327–359. [Google Scholar] [CrossRef]
- Liptow, C.; Tillman, A.-M. A Comparative Life Cycle Assessment Study of Polyethylene Based on Sugarcane and Crude Oil. J. Ind. Ecol. 2012, 16, 420–435. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a biocomposite based on green polyethylene biopolymer and eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Ferrero, B.; Fombuena, V.; Fenollar, O.; Boronat, T.; Balart, R. Development of natural fiber-reinforced plastics (NFRP) based on biobased polyethylene and waste fibers from Posidonia oceanica seaweed. Polym. Compos. 2015, 36, 1378–1385. [Google Scholar] [CrossRef]
- Filgueira, D.; Holmen, S.; Melbo, J.K.; Moldes, D.; Echtermeyer, A.T.; Chinga-Carrasco, G. 3D Printable Filaments Made of Biobased Polyethylene Biocomposites. Polymers 2018, 10, 314. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Biobased Polyamides: Recent Advances in Basic and Applied Research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef]
- Jiang, Y.; Loos, K. Enzymatic Synthesis of Biobased Polyesters and Polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef]
- Jasinska, L.; Villani, M.; Wu, J.; van Es, D.; Klop, E.; Rastogi, S.; Koning, C.E. Novel, Fully Biobased Semicrystalline Polyamides. Macromolecules 2011, 44, 3458–3466. [Google Scholar] [CrossRef]
- Ha Thi Hoang, N.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kimura, S.; Kasuya, K.-i. Synthesis and Verification of Biobased Terephthalic Acid from Furfural. Sci. Rep. 2015, 5, 8249. [Google Scholar] [CrossRef]
- Neatu, F.; Culica, G.; Florea, M.; Parvulescu, V.I.; Cavani, F. Synthesis of Terephthalic Acid by p-Cymene Oxidation using Oxygen: Toward a More Sustainable Production of Bio-Polyethylene Terephthalate. Chemsuschem 2016, 9, 3102–3112. [Google Scholar] [CrossRef]
- Yasuda, M.; Miyabo, A. Polyamide Derived from Castor Oil. Sen-I Gakkaishi 2010, 66, P137–P142. [Google Scholar] [CrossRef]
- Moran, C.S.; Barthelon, A.; Pearsall, A.; Mittal, V.; Dorgan, J.R. Biorenewable blends of polyamide-4,10 and polyamide-6,10. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Balart, R.; Torres-Giner, S. Evaluation of the engineering performance of different bio-based aliphatic homopolyamide tubes prepared by profile extrusion. Polym. Test. 2017, 61, 421–429. [Google Scholar] [CrossRef]
- Welle, F.; Bayer, F.; Franz, R. Quantification of the Sorption Behavior of Polyethylene Terephthalate Polymer versus PET/PA Polymer Blends towards Organic Compounds. Packag. Technol. Sci. 2012, 25, 341–349. [Google Scholar] [CrossRef]
- Fabia, J.; Gawlowski, A.; Graczyk, T.; Slusarczyk, C. Changes of crystalline structure of poly(ethylene terephthalate) fibers in flame retardant finishing process. Polimery 2014, 59, 557–561. [Google Scholar] [CrossRef]
- Kuciel, S.; Kuznia, P.; Jakubowska, P. Properties of composites based on polyamide 10.10 reinforced with carbon fibers. Polimery 2016, 61, 106–112. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Szostak, M.; Bak, T.; Trzeciak, M. The influence of processing conditions on the mechanical properties and structure of poly(ethylene terephthalate) self-reinforced composites. J. Thermoplast. Compos. Mater. 2016, 29, 1194–1209. [Google Scholar] [CrossRef]
- Cook, W.D.; Moad, G.; Fox, B.; VanDeipen, G.; Zhang, T.; Cser, F.; McCarthy, L. Morphology-property relationships in ABS/PET blends. 2. Influence of processing conditions on structure and properties. J. Appl. Polym. Sci. 1996, 62, 1709–1714. [Google Scholar] [CrossRef]
- Bartolotta, A.; Di Marco, G.; Farsaci, F.; Lanza, M.; Pieruccini, M. DSC and DMTA study of annealed cold-drawn PET: A three phase model interpretation. Polymer 2003, 44, 5771–5777. [Google Scholar] [CrossRef]
- Chen, Z.; Jenkins, M.J.; Hay, J.N. Annealing of poly (ethylene terephthalate). Eur. Polym. J. 2014, 50, 235–242. [Google Scholar] [CrossRef]
- Chiou, K.C.; Chang, F.C. Reactive compatibilization of polyamide-6 (PA 6)/polybutylene terephthalate (PBT) blends by a multifunctional epoxy resin. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 23–33. [Google Scholar] [CrossRef]
- Samios, C.K.; Kalfoglou, N.K. Compatibilization of poly(ethylene terephthalate)/polyamide-6 alloys: Mechanical, thermal and morphological characterization. Polymer 1999, 40, 4811–4819. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Liu, Y.X.; Zhao, C.H. Morphology and properties of PET/PA-6/E-44 blends. J. Appl. Polym. Sci. 1998, 69, 1505–1515. [Google Scholar] [CrossRef]
- Ferreira, C.T.; da Fonseca, J.B.; Saron, C. Recycling of Wastes from Poly(ethylene tereftalate) (PET) and Polyamide (PA) by Reactive Extrusion for Preparation of Polymeric Blends. Polim.-Cienc. E Tecnol. 2011, 21, 118–122. [Google Scholar] [CrossRef]
- Yan, Y.; Gooneie, A.; Ye, H.; Deng, L.; Qiu, Z.; Reifler, F.A.; Hufenus, R. Morphology and Crystallization of Biobased Polyamide 56 Blended with Polyethylene Terephthalate. Macromol. Mater. Eng. 2018, 303, 1800214. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarria, G.; Ignacio Eguiazabal, J. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; Garcia-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Xue, B.; He, H.; Zhu, Z.; Li, J.; Huang, Z.; Wang, G.; Chen, M.; Zhan, Z. A Facile Fabrication of High Toughness Poly(lactic Acid) via Reactive Extrusion with Poly(butylene Succinate) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate. Polymers 2018, 10, 1401. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.-L.; Qu, J.-P. Super-Toughened Poly(lactic Acid) with Poly(epsilon-caprolactone) and Ethylene-Methyl Acrylate-Glycidyl Methacrylate by Reactive Melt Blending. Polymers 2019, 11, 771. [Google Scholar] [CrossRef]
- Jesus Garcia-Campo, M.; Boronat, T.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Manufacturing and Characterization of Toughened Poly(lactic acid) (PLA) Formulations by Ternary Blends with Biopolyesters. Polymers 2018, 10, 3. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Uribe-Calderon, J.; Diaz-Arriaga, C. The effects of carbon nanotubes, blend composition and glycidyl methacrylate-grafted polypropylene compatibilizer on the morphology, mechanical and electrical properties of polypropylene-polyamide 6 blends. Polym. Bull. 2017, 74, 1573–1593. [Google Scholar] [CrossRef]
- Shin, B.Y.; Ha, M.H.; Han, D.H. Morphological, Rheological, and Mechanical Properties of Polyamide 6/Polypropylene Blends Compatibilized by Electron-Beam Irradiation in the Presence of a Reactive Agent. Materials 2016, 9, 342. [Google Scholar] [CrossRef]
- Li, D.; Song, S.; Li, C.; Cao, C.; Sun, S.; Zhang, H. Compatibilization effect of MMA-co-GMA copolymers on the properties of polyamide 6/Poly(vinylidene fluoride) blends. J. Polym. Res. 2015, 22, 102. [Google Scholar] [CrossRef]
- Lima, M.S.; Matias, A.A.; Costa, J.R.C.; Fonseca, A.C.; Coelho, J.F.J.; Serra, A.C. Glycidyl methacrylate-based copolymers as new compatibilizers for polypropylene/polyethylene terephthalate blends. J. Polym. Res. 2019, 26, 127. [Google Scholar] [CrossRef]
- Pietrasanta, Y.; Robin, J.J.; Torres, N.; Boutevin, B. Reactive compatibilization of HDPE/PET blends by glycidyl methacrylate functionalized polyolefins. Macromol. Chem. Phys. 1999, 200, 142–149. [Google Scholar] [CrossRef]
- McLauchlin, A.R.; Ghita, O.R. Studies on the thermal and mechanical behavior of PLA-PET blends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Palma-Ramirez, D.; Dominguez-Crespo, M.A.; Del Angel-Lopez, D.; de la Fuente, D. Comparative assessment of miscibility and degradability on PET/PLA and PET/chitosan blends. Eur. Polym. J. 2014, 61, 285–299. [Google Scholar] [CrossRef]
- Carrot, C.; Mbarek, S.; Jaziri, M.; Chalamet, Y.; Raveyre, C.; Prochazka, F. Immiscible blends of PC and PET, current knowledge and new results: Rheological properties. Macromol. Mater. Eng. 2007, 292, 693–706. [Google Scholar] [CrossRef]
- Jazani, O.M.; Rastin, H.; Formela, K.; Hejna, A.; Shahbazi, M.; Farkiani, B.; Saeb, M.R. An investigation on the role of GMA grafting degree on the efficiency of PET/PP-g-GMA reactive blending: Morphology and mechanical properties. Polym. Bull. 2017, 74, 4483–4497. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crops Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Rayon, E.; Carbonell-Verdu, A.; Lopez-Martinez, J.; Balart, R. Improvement of the compatibility between poly(3-hydroxybutyrate) and poly(8-caprolactone) by reactive extrusion with dicumyl peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef]
- Jiang, S.; Mi, R.; Yun, R.; Qi, S.; Zhang, X.; Lu, Y.; Matejka, V.; Peikertova, P.; Tokarsky, J. Structure and properties of kaolinite intercalated with potassium acetate and their nanocomposites with polyamide 1010. J. Thermoplast. Compos. Mater. 2017, 30, 971–985. [Google Scholar] [CrossRef]
- Serhatkulu, T.; Erman, B.; Bahar, I.; Fakirov, S.; Evstatiev, M.; Sapundjieva, D. Dynamic-mechanical study of amorphous phases in poly(ethylene terephthalate)/nylon-6 blends. Polymer 1995, 36, 2371–2377. [Google Scholar] [CrossRef]










| Property | bioPET | bioPA1010 |
|---|---|---|
| Grade | BioPET 001 | NP BioPA1010-201 |
| wt % bio-based | 30 | 100 |
| Melt temperature (°C) | 240–260 | 190–210 |
| Density (g cm−3) | 1.3–1.4 | 1.05 |
| Intrinsic viscosity (mL·g−1) | 75–79 | 84–90 * |
| Label | bioPET (wt %) | bioPA (wt %) | Xibond™ (phr)* | Bio-based content (wt %) |
|---|---|---|---|---|
| PET100 | 100 | - | - | 30.0 |
| PET90 | 90 | 10 | - | 37.0 |
| PET80 | 80 | 20 | - | 44.0 |
| PET70 | 70 | 30 | - | 51.0 |
| PET70Xibond1 | 70 | 30 | 1 | 50.5 |
| PET70Xibond3 | 70 | 30 | 3 | 49.5 |
| PET70Xibond5 | 70 | 30 | 5 | 48.6 |
| Code | σb (MPa) | εy (%) | Shore D | Impact Strength (kJ·m−2) |
|---|---|---|---|---|
| PET100 | 46.7 ± 2.3 | 3.87 ± 0.30 | 75 ± 2.5 | 23.1 ± 4.4 |
| PET90 | 41.5 ± 4.6 | 4.30 ± 0.39 | 75 ± 2.5 | 27.0 ± 3.8 |
| PET80 | 42.8 ± 2.5 | 4.71 ± 1.04 | 75 ± 2.8 | 30.3 ± 3.6 |
| PET70 | 41.4 ± 0.6 | 4.80 ± 0.40 | 75 ± 2.9 | 40.5 ± 9.9 |
| PET70Xibond1 | 41.3 ± 0.8 | 5.01 ± 0.69 | 73 ± 2.9 | 42.9 ± 2.7 |
| PET70Xibond3 | 47.1 ± 1.1 | 6.09 ± 0.86 | 75 ± 2.3 | 43.4 ± 1.6 |
| PET70Xibond5 | 40.6 ± 4.5 | 6.63 ± 1.94 | 74 ± 2.5 | 44.6 ± 3.9 |
| Code | bioPET | bioPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg | Tcc | ΔHcc (J g−1) | ΔHm (J g−1) | Tm | χc (%) | ΔHm (J g−1) | Tm | χc (%) | |
| PET100 | 75.2 | 133.2 | 27.6 | −31.8 | 248.2 | 22.7 | - | - | - |
| PET90 | 75.8 | 121.9 | 11.7 | −27.5 | 248.8 | 21.8 | −2.4 | 202.5 | 9.8 |
| PET80 | 75.6 | - | - | −25.3 | 247.6 | 22.6 | −4.9 | 202.4 | 10.0 |
| PET70 | 75.4 | - | - | −19.5 | 246.7 | 19.9 | −12.4 | 201.9 | 16.9 |
| PET70Xibond1 | 78.3 | - | - | −19.1 | 247.9 | 19.7 | −8.8 | 202.4 | 12.1 |
| PET70Xibond3 | 78.6 | - | - | −18.4 | 247.6 | 19.3 | −8.7 | 202.5 | 12.2 |
| PET70Xibond5 | 77.3 | - | - | −16.20 | 248.1 | 17.3 | −7.1 | 203.1 | 10.2 |
| Code | Tmax | |
|---|---|---|
| PET100 | 382.6 | 452.6 |
| PET90 | 392.8 | 450.4 |
| PET80 | 393.2 | 443.7 |
| PET70 | 397.4 | 441.1 |
| PET70Xibond1 | 399.3 | 442.6 |
| PET70Xibond3 | 403.7 | 446.9 |
| PET70Xibond5 | 394.7 | 441.8 |
| Code | CLTE (μm·m−1·K−1)* |
|---|---|
| PET100 | 152.4 ± 12.2 |
| PET90 | 162.4 ± 10.4 |
| PET80 | 262.1 ± 15.1 |
| PET70 | 347.3 ± 22.9 |
| PET70Xibond1 | 325.8 ± 45.0 |
| PET70Xibond3 | 163.2 ± 19.3 |
| PET70Xibond5 | 172.4 ± 22.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorda, M.; Montava-Jorda, S.; Balart, R.; Lascano, D.; Montanes, N.; Quiles-Carrillo, L. Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer. Polymers 2019, 11, 1331. https://doi.org/10.3390/polym11081331
Jorda M, Montava-Jorda S, Balart R, Lascano D, Montanes N, Quiles-Carrillo L. Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer. Polymers. 2019; 11(8):1331. https://doi.org/10.3390/polym11081331
Chicago/Turabian StyleJorda, Maria, Sergi Montava-Jorda, Rafael Balart, Diego Lascano, Nestor Montanes, and Luis Quiles-Carrillo. 2019. "Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer" Polymers 11, no. 8: 1331. https://doi.org/10.3390/polym11081331
APA StyleJorda, M., Montava-Jorda, S., Balart, R., Lascano, D., Montanes, N., & Quiles-Carrillo, L. (2019). Functionalization of Partially Bio-Based Poly(Ethylene Terephthalate) by Blending with Fully Bio-Based Poly(Amide) 10,10 and a Glycidyl Methacrylate-Based Compatibilizer. Polymers, 11(8), 1331. https://doi.org/10.3390/polym11081331