Electric Heating Behavior of Reduced Oxide Graphene/Carbon Nanotube/Natural Rubber Composites with Macro-Porous Structure and Segregated Filler Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Macro-Porous rGO/CNT/NR Composites with a Segregated Network
2.3. Characterization
3. Results and Discussion
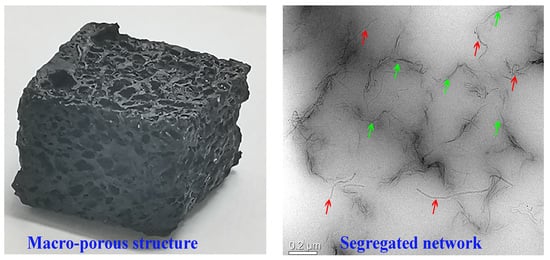
3.1. Structure and Morphology of Macro-Porous GCR Composites
3.2. Density of Macro-Porous GCR Composites
3.3. Electrical Conductivity of Macro-Porous GCR Composites
3.4. Electric Heating Behavior of Macro-Porous GCR Composites
3.5. Compression Stress of Macro-Porous GCR Composites
3.6. Thermal Stability of Macro-Porous GCR Composites
3.7. Glass-Transition Temperature of Macro-Porous GCR Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.; Han, D.; Choi, S.; Kim, Y.; Kwak, J. Flexible transparent film heaters using a ternary composite of silver nanowire, conducting polymer, and conductive oxide. RSC Adv. 2019, 9, 5731–5737. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Kang, S.; Ma, J.; Shao, L.; Wei, A.; Liang, C.; Gu, J.; Yang, B.; Dong, D.; Wei, L.; et al. High-Performance and Rapid-Response Electrical Heaters Based on Ultraflexible, Heat-Resistant, and Mechanically Strong Aramid Nanofiber/Ag Nanowire Nanocomposite Papers. ACS Nano 2019, 13, 7578–7590. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Jiang, X.; Zhao, W.; Wang, F.; Lan, W.; Xie, E.; Han, W. A facile method to prepare transparent and stretchable epidermal thin film heaters. Compos. Sci. Technol. 2018, 168, 460–466. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, Y.; Li, Y. Electric heating behavior of flexible graphene/natural rubber conductor with self-healing conductive network. Mater. Lett. 2017, 192, 115–118. [Google Scholar] [CrossRef]
- Yagubov, V.; Shchegolkov, A.; Shchegolkov, A.; Tkachev, A.; Sukhorukov, A. Flat electric heaters with the effect of self-regulation based on nanomodified polymer composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 693, 12018. [Google Scholar] [CrossRef] [Green Version]
- Pyo, K.-H.; Kim, J.-W. Transparent and mechanically robust flexible heater based on compositing of Ag nanowires and conductive polymer. Compos. Sci. Technol. 2016, 133, 7–14. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, P.; Ge, H. Research on PTC electric heater detection bench. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 32033. [Google Scholar] [CrossRef]
- Jeong, Y.G.; Jeon, G.W. Microstructure and performance of multiwalled carbon nanotube/m-aramid composite films as electric heating elements. ACS Appl. Mater. Interfaces 2013, 5, 6527–6534. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Zhang, S.; Yin, R.; Liu, X.; He, Y.; Dai, K.; Shan, C.; Guo, J.; Liu, C.; et al. Electrically conductive polymer composites for smart flexible strain sensors: A critical review. J. Mater. Chem. C 2018, 6, 12121–12141. [Google Scholar] [CrossRef]
- Zhan, Y.; Lago, E.; Santillo, C.; Del Río Castillo, A.E.; Hao, S.; Buonocore, G.G.; Chen, Z.; Xia, H.; Lavorgna, M.; Bonaccorso, F. An anisotropic layer-by-layer carbon nanotube/boron nitride/rubber composite and its application in electromagnetic shielding. Nanoscale 2020, 12, 7782–7791. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Zhou, H.; Nie, M.; Wang, Q.; Hua, Z. Polyurethane/carbon fiber composite tubular electrode featuring three-dimensional interpenetrating conductive network. Carbon 2018, 139, 999–1009. [Google Scholar] [CrossRef]
- Cheng, C.; Ke, K.-C.; Yang, S.-Y. Application of graphene–polymer composite heaters in gas-assisted micro hot embossing. RSC Adv. 2017, 7, 6336–6344. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Lavorgna, M.; Buonocore, G.; Xia, H. Enhancing electrical conductivity of rubber composites by constructing interconnected network of self-assembled graphene with latex mixing. J. Mater. Chem. 2012, 22, 10464. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Nakanishi, K.; Huang, J. Superhydrophobic ultraflexible triple-network graphene/polyorganosiloxane aerogels for a high-performance multifunctional temperature/strain/pressure sensing array. Chem. Mater. 2019, 31, 6276–6285. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Hao, S.; Cheng, Y.; Zhan, Y.; Zhang, C.; Meng, Y.; Xie, Q.; Xia, H. Well-aligned MXene/chitosan films with humidity response for high-performance electromagnetic interference shielding. Carbohyd. Polym. 2020, 243, 116467. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.-B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.-Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Patel, M.; Chauhan, K.R.; Kim, J.; Kim, J.-W.; Lim, D. AgNWs networks for high-performing transparent heaters by using NiO window layer. Sens. Actuators A Phys. 2017, 267, 8–13. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Wang, Y.; Zhai, T.; Verdolotti, L.; Buonocore, G.G.; Lavorgna, M.; Xia, H. Nanocomposite polymeric materials with 3D graphene-based architectures: From design strategies to tailored properties and potential applications. Prog. Polym. Sci. 2019, 89, 213–249. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, H.-B.; Liu, J.; Xie, X.; Yang, R.; Li, Y.; Hong, S.; Yu, Z.-Z. Highly conductive transition metal carbide/carbonitride(MXene)@polystyrene nanocomposites fabricated by electrostatic assembly for highly efficient electromagnetic interference shielding. Adv. Funct. Mater. 2017, 27, 1702807. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Wu, S.; Tang, Z.; Guo, B.; Zhang, L. A green method for preparing conductive elastomer composites with interconnected graphene network via Pickering emulsion templating. Chem. Eng. J. 2018, 342, 112–119. [Google Scholar] [CrossRef]
- Hao, S.; Wang, J.; Lavorgna, M.; Fei, G.; Wang, Z.; Xia, H. Constructing 3D graphene network in rubber nanocomposite via liquid-phase redispersion and self-assembly. ACS Appl. Mater. Interfaces 2020, 12, 9682–9692. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, J.; Zhang, K.; Li, Y.; Meng, Y.; Yan, N.; Wei, W.; Peng, F.; Xia, H. Fabrication of a flexible electromagnetic interference shielding Fe3O4@reduced graphene oxide/natural rubber composite with segregated network. Chem. Eng. J. 2018, 344, 184–193. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Jia, L.-C.; Yan, D.-X.; Gao, J.-F.; Zhang, X.-P.; Ren, P.-G.; Li, Z.-M. Simultaneously improved electromagnetic interference shielding and mechanical performance of segregated carbon nanotube/polypropylene composite via solid phase molding. Compos. Sci. Technol. 2018, 156, 87–94. [Google Scholar] [CrossRef]
- Sharif, F.; Arjmand, M.; Moud, A.A.; Sundararaj, U.; Roberts, E.P.L. Segregated hybrid poly(methyl methacrylate)/graphene/magnetite nanocomposites for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2017, 9, 14171–14179. [Google Scholar] [CrossRef]
- Yu, C.; Kim, Y.S.; Kim, D.; Grunlan, J.C. Thermoelectric behavior of segregated-network polymer nanocomposites. Nano Lett. 2008, 8, 4428–4432. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Fu, D.; Wang, Z.; Gan, X.; Lu, X.; Yang, L.; Xia, H. Realizing crack diagnosing and self-healing by electricity with a dynamic crosslinked flexible polyurethane composite. Adv. Sci. 2018, 5, 1800101. [Google Scholar] [CrossRef]
- Vahidifar, A.; Nouri Khorasani, S.; Park, C.B.; Naguib, H.E.; Khonakdar, H.A. Fabrication and characterization of closed-cell rubber foams based on natural rubber/carbon black by one-step foam processing. Ind. Eng. Chem. Res. 2016, 55, 2407–2416. [Google Scholar] [CrossRef]
- Yan, H.; Wang, K.; Zhao, Y. Fabrication of silicone rubber foam with tailored porous structures by supercritical CO2. Macromol. Mater. Eng. 2017, 302, 1600377. [Google Scholar] [CrossRef]
- Liu, C.; Wu, C.; Hao, C.; Liu, P.; Guo, X.; Zhang, Y.; Huang, Y. Electrical conductivity transformation mechanism of GNPs/CB/SR nanocomposite foams. J. Appl. Polym. Sci. 2018, 135, 45996. [Google Scholar] [CrossRef]
- Ameli, A.; Nofar, M.; Park, C.B.; Pötschke, P.; Rizvi, G. Polypropylene/carbon nanotube nano/microcellular structures with high dielectric permittivity, low dielectric loss, and low percolation threshold. Carbon 2014, 71, 206–217. [Google Scholar] [CrossRef]
- Zhan, Y.; Oliviero, M.; Wang, J.; Sorrentino, A.; Buonocore, G.G.; Sorrentino, L.; Lavorgna, M.; Xia, H.; Iannace, S. Enhancing the EMI shielding of natural rubber-based supercritical CO2 foams by exploiting their porous morphology and CNT segregated networks. Nanoscale 2019, 11, 1011–1020. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Mark, L.H.; Shaayegan, V.; Wang, G.; Li, H.; Zhao, G.; Park, C.B. Ultralow-threshold and lightweight biodegradable porous PLA/MWCNT with segregated conductive networks for high-performance thermal insulation and electromagnetic interference shielding applications. ACS Appl. Mater. Interfaces 2018, 10, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wu, J.; Xia, H.; Yan, N.; Fei, G.; Yuan, G. Dispersion and exfoliation of graphene in rubber by an ultrasonically-assisted latex mixing and in situ reduction process. Macromol. Mater. Eng. 2011, 296, 590–602. [Google Scholar] [CrossRef]
- Zhan, Y.; Yan, N.; Li, Y.; Meng, Y.; Wang, J.; Zhang, N.; Yu, Q.; Xia, H. Fabrication of graphene millimeter-vortex ring with excellent absorption via solution dripping and in-situ reduction method. Chem. Eng. J. 2017, 327, 142–149. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, Y.; Yan, N.; Li, Y.; Wei, D.; Tao, X. Enhancing electrochemical performance of Fe3O4/graphene hybrid aerogel with hydrophilic polymer. J. Appl. Polym. Sci. 2017, 134, 45566. [Google Scholar] [CrossRef]
- Huang, Z.-D.; Zhang, B.; Oh, S.-W.; Zheng, Q.-B.; Lin, X.-Y.; Yousefi, N.; Kim, J.-K. Self-assembled reduced graphene oxide/carbon nanotube thin films as electrodes for supercapacitors. J. Mater. Chem. 2012, 22, 3591. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, Z.; Gui, X.; Tang, Z.; Zou, M.; Cao, A. Carbon nanotube sponges, aerogels, and hierarchical composites: Synthesis, properties, and energy applications. Adv. Energy Mater. 2016, 6, 1600554. [Google Scholar] [CrossRef]
- Jia, L.-C.; Yan, D.-X.; Yang, Y.; Zhou, D.; Cui, C.-H.; Bianco, E.; Lou, J.; Vajtai, R.; Li, B.; Ajayan, P.M.; et al. High strain tolerant EMI shielding using carbon nanotube network stabilized rubber composite. Adv. Mater. Technol. 2017, 2, 1700078. [Google Scholar] [CrossRef]
- Pilipović, A.; Ilinčić, P.; Petruša, J.; Domitran, Z. Influence of polymer composites and memory foam on energy absorption in vehicle application. Polymers 2020, 12, 1222. [Google Scholar] [CrossRef]
- Jia, Y.; Bai, S.; Park, C.B.; Wang, Q. Effect of boric acid on the foaming properties and cell structure of poly(vinyl alcohol) foam prepared by supercritical-CO2 thermoplastic extrusion foaming. Ind. Eng. Chem. Res. 2017, 56, 6655–6663. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Jia, Y.; Bai, S. Polysulfone foam with high expansion ratio prepared by supercritical carbon dioxide assisted molding foaming method. RSC Adv. 2018, 8, 2880–2886. [Google Scholar] [CrossRef] [Green Version]
- Bilent, S.; Nhung Dinh, T.H.; Martincic, E.; Joubert, P.-Y. Influence of the porosity of polymer foams on the performances of capacitive flexible pressure sensors. Sensors 2019, 19, 1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.-C.; Yan, D.-X.; Cui, C.-H.; Jiang, X.; Ji, X.; Li, Z.-M. Electrically conductive and electromagnetic interference shielding of polyethylene composites with devisable carbon nanotube networks. J. Mater. Chem. C 2015, 3, 9369–9378. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Mondal, S.; Ganguly, S.; Rahaman, M.; Aldalbahi, A.; Chaki, T.K.; Khastgir, D.; Das, N.C. A strategy to achieve enhanced electromagnetic interference shielding at low concentration with a new generation of conductive carbon black in a chlorinated polyethylene elastomeric matrix. Phys. Chem. Chem. Phys. 2016, 18, 24591–24599. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Jeong, Y.G. Regenerated cellulose/multiwalled carbon nanotube composite films with efficient electric heating performance. Carbohyd. Polym. 2015, 133, 456–463. [Google Scholar] [CrossRef]
- Alam, M.K.; Islam, M.T.; Mina, M.F.; Gafur, M.A. Structural, mechanical, thermal, and electrical properties of carbon black reinforced polyester resin composites. J. Appl. Polym. Sci. 2014, 131, 40421. [Google Scholar] [CrossRef]








| Sample | GO (g) | rGO (g) | CNT (g) | H2O (g) | NRL (60%) | N2H4(mL) |
|---|---|---|---|---|---|---|
| G0.5C0.5R | 0.25 | 0.15 | 0.15 | 100 | 50 | 0.25 |
| G0.5C2.5R | 0.08 | 0.05 | 0.25 | 100 | 16.67 | 0.08 |
| G1.5C1.5R | 0.25 | 0.15 | 0.15 | 100 | 16.67 | 0.25 |
| G2.5C0.5R | 0.42 | 0.25 | 0.05 | 100 | 16.67 | 0.42 |
| G3C3R | 0.5 | 0.30 | 0.30 | 200 | 16.67 | 0.50 |
| Sample | Voltage (V) | τg (s) | hr+c (mW/°C) |
|---|---|---|---|
| G2.5C0.5R | 15 | 35.0 | 3.4 |
| G1.5C1.5R | 15 | 32.6 | 3.0 |
| G0.5C2.5R | 15 | 20.4 | 2.4 |
| G3C3R | 15 | 23.3 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Li, Y.; Meng, Y.; Xie, Q.; Lavorgna, M. Electric Heating Behavior of Reduced Oxide Graphene/Carbon Nanotube/Natural Rubber Composites with Macro-Porous Structure and Segregated Filler Network. Polymers 2020, 12, 2411. https://doi.org/10.3390/polym12102411
Zhan Y, Li Y, Meng Y, Xie Q, Lavorgna M. Electric Heating Behavior of Reduced Oxide Graphene/Carbon Nanotube/Natural Rubber Composites with Macro-Porous Structure and Segregated Filler Network. Polymers. 2020; 12(10):2411. https://doi.org/10.3390/polym12102411
Chicago/Turabian StyleZhan, Yanhu, Yuchao Li, Yanyan Meng, Qian Xie, and Marino Lavorgna. 2020. "Electric Heating Behavior of Reduced Oxide Graphene/Carbon Nanotube/Natural Rubber Composites with Macro-Porous Structure and Segregated Filler Network" Polymers 12, no. 10: 2411. https://doi.org/10.3390/polym12102411
APA StyleZhan, Y., Li, Y., Meng, Y., Xie, Q., & Lavorgna, M. (2020). Electric Heating Behavior of Reduced Oxide Graphene/Carbon Nanotube/Natural Rubber Composites with Macro-Porous Structure and Segregated Filler Network. Polymers, 12(10), 2411. https://doi.org/10.3390/polym12102411