Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques
Abstract
:1. Introduction
2. Experimental
2.1. Materials
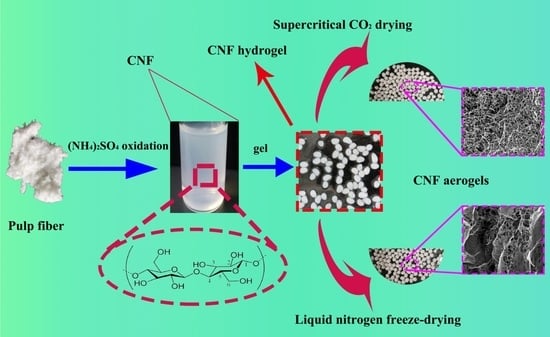
2.2. Preparation of CNF
2.3. Preparation of CNF Hydrogels
2.4. Preparation of CNF Aerogels by Supercritical CO2 Drying
2.5. Preparation of CNF Aerogels by Liquid Nitrogen Freeze-Drying
2.6. Characterization
3. Results and Discussion
3.1. FT-IR, TEM, and XRD of CNF
3.2. Morphological Characterization
3.3. Analysis of Shrinkage, BET, Pore Volume, and Density
3.4. Compression Strength
3.5. Analysis of N2 Adsorption/Desorption Isotherms
3.6. Analysis of the Pore Size Distributions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Huang, X.; Feng, J.; Qi, F.; Dianyu, E.; Jiang, Y.; Li, L.; Xiong, S.; Feng, J. Structure, compression and thermally insulating properties of cellulose diacetate-based aerogels. Mater. Des. 2020, 189, 108502. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Jia, Z.; Wang, L.; Guo, X.; Zhao, B.; Zhang, R. Exceptionally porous three-dimensional architectural nanostructure derived from CNTs/graphene aerogel towards the ultra-wideband EM absorption. Compos. Part B Eng. 2020, 196, 108122. [Google Scholar] [CrossRef]
- Sun, X.; Ji, S.; Wang, M.; Dou, J.; Yang, Z.; Qiu, H.; Kou, S.; Ji, Y.; Wang, H. Fabrication of porous TiO2-RGO hybrid aerogel for high-eciency, visible-light photodegradation of dyes. J. Alloys Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- Kaya, G.G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Gu, W.; Zhao, H.; Zheng, J.; Zhang, B.; Ji, G. Cellulose-chitosan framework/polyailine hybrid aerogel toward thermal insulation and microwave absorbing application. Chem. Eng. J. 2020, 395, 125190. [Google Scholar] [CrossRef]
- Javaid, A.; Irfan, M. Multifunctional structural supercapacitors based on graphene nanoplatelets/carbon aerogel composite coated carbon fiber electrodes. Mater. Res. Express 2019, 6, 016310. [Google Scholar] [CrossRef]
- Niu, F.; Wu, N.; Yu, J.; Ma, X. Gelation, flame retardancy, and physical properties of phosphorylated microcrystalline cellulose aerogels. Carbohydr. Polym. 2020, 242, 116422. [Google Scholar] [CrossRef]
- Tang, C.; Brodie, P.; Li, Y.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, H.; Wang, X. Adjusting the pore size of nano-cellulose aerogel by heat treatment in the gel stage. Mater. Res. Express 2019, 6, 075027. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81. [Google Scholar] [CrossRef]
- Shang, Q.; Chen, J.; Yang, X.; Liu, C.; Hu, Y.; Zhou, Y. Fabrication and oil absorbency of superhydrophobic magnetic cellulose aerogels. J. For. Eng. 2019, 4, 6. [Google Scholar]
- Zhang, X.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.I.B. Biohybrid hydrogel and aerogel from self-assembled nanocellulose and nanochitin as a high-efficiency adsorbent for water purification. ACS Appl. Mater. Interfaces 2019, 11, 46714. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Li, J.; Tan, S.; Chen, P.; Xu, Z. Preparation and properties of nanocellulose-based oil-absorbing aerogels. J. For. Eng. 2019, 4, 74. [Google Scholar]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of nanocellulose aerogels and hydrogels. Chem. Mater. 2017, 29, 4609. [Google Scholar] [CrossRef]
- Zhu, W.; Ji, M.; Chen, F.; Wang, Z.; Chen, W.; Xue, Y.; Zhang, Y. Formaldehyde-free resin impregnated paper reinforced with cellulose nanocrystal (CNC): Formulation and property analysis. J. Appl. Polym. Sci. 2020, 137, 48931. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Xu, D. Synthesis and characterization of a xonotlite fibers–silica aerogel composite by ambient pressure drying. J. Porous Mater. 2018, 25, 1417. [Google Scholar] [CrossRef]
- Missfeldt, F.; Gurikov, P.; Loelsberg, W.; Weinrich, D.; Lied, F.; Fricke, M.; Smirnova, I. Continuous supercritical drying of aerogel particles: Proof of concept. Ind. Eng. Chem. Res. 2020, 59, 11284. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115. [Google Scholar] [CrossRef]
- Kim, C.H.; Youn, H.J.; Lee, H.L. Preparation of surface-charged CNF aerogels and investigation of their ion adsorption properties. Cellulose 2015, 22, 3715. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Guo, Y.; Niu, Q.; Fang, H.; Zhang, L.; Zhang, Y.; Wang, L.; Zhou, L. Effects of chitin nanofibers on the microstructure and properties of cellulose nanofibers/chitin nanofibers composite aerogels. Cellulose 2018, 25, 4591. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Kondo, H.; Takagi, H. Cellulose nanofiber aerogel production and applications. J. Reinf. Plast. Compos. 2013, 32, 1547. [Google Scholar] [CrossRef]
- Ciftci, D.; Ubeyitogullari, A.; Huerta, R.R.; Ciftci, O.N.; Flores, R.A.; Saldana, M.D.A. Lupin hull cellulose nanofiber aerogel preparation by supercritical CO2 and freeze drying. J. Supercrit. Fluids 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater. Lett. 2016, 183, 179. [Google Scholar] [CrossRef]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of nanofibrous structure in biopolymer aerogel during supercritical CO2 processing: The case of chitosan aerogel. Biomacromolecules 2019, 20, 2051. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Natural Aerogels Production by Supercritical Gel Drying. Chem. Eng. Trans. 2015, 43, 739. [Google Scholar]
- Wan, C.; Li, J. Graphene oxide/cellulose aerogels nanocomposite: Preparation, pyrolysis, and application for electromagnetic interference shielding. Carbohydr. Polym. 2016, 150, 172. [Google Scholar] [CrossRef] [PubMed]
- Shamskar, K.R.; Heidari, H.; Rashidi, A. Study on nanocellulose properties processed using different methods and their aerogels. J. Polym. Environ. 2019, 27, 1418. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Wang, X.; Liu, S.; Yao, Y. Characterization of the nano-cellulose aerogel from mixing CNF and CNC with different ratio. Mater. Lett. 2018, 229, 103. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; He, C.; Lu, Z.; Huang, J.; Chen, Z. Facilitated fabrication of high strength silica aerogels using cellulose nanofibrils as scaffold. Carbohydr. Polym. 2016, 147, 89. [Google Scholar] [CrossRef] [PubMed]
- Stanislas, T.T.; Tendo, J.F.; Ojo, E.B.; Ngasoh, O.F.; Onwualu, P.A.; Njeugna, E.; Junior, H.S. Production and characterization of pulp and nanofibrillated cellulose from selected tropical plants. J. Nat. Fibers 2020, 1–17. [Google Scholar] [CrossRef]
- Onkarappa, H.S.; Prakash, G.K.; Pujar, G.H.; Kumar, C.R.R.; Radha, V.; Betageri, V.S. Facile synthesis and characterization of nanocellulose from Zea mays husk. Polym. Compos. 2020, 41, 3153. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, H.; Liu, S.; Yao, Y. Characterization of CNF/CNC composite aerogel. J. For. Eng. 2018, 3, 101. [Google Scholar]
- Wang, X.; Zhang, Y.; Wang, S.; Jiang, H.; Liu, S.; Yao, Y.; Zhang, T.; Li, Q. Synthesis and characterization of amine-modified spherical nanocellulose aerogels. J. Mater. Sci. 2018, 53, 13304. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Feng, X.; Zhang, Q.; Labbe, N.; Kim, K.; Huang, J.; Ragauskas, A.J.; Wang, S.; Zhang, Y. Isolation and characterization of lignocellulosic nanofibers from four kinds of organosolv-fractionated lignocellulosic materials. Wood Sci. Technol. 2020, 54, 503. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Jiang, H.; Wang, X.; Zhang, T.; Yao, Y. High CO2 adsorption by amino-modified bio-spherical cellulose nanofibres aerogels. Environ. Chem. Lett. 2018, 16, 605. [Google Scholar] [CrossRef]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterization of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121. [Google Scholar] [CrossRef]







| Samples | Shrinkage (%) | BET (m2/g) | Pore Volume (cm3/g) | Density (g/cm3) |
|---|---|---|---|---|
| 1.5-SCD-CNF aerogel | 5.56 | 296.23 | 0.7196 | 0.0201 |
| 2.5-SCD-CNF aerogel | 4.12 | 324.36 | 0.6112 | 0.0263 |
| 3.5-SCD-CNF aerogel | 4.35 | 330.88 | 0.5024 | 0.0362 |
| Samples | Shrinkage (%) | BET (m2/g) | Pore Volume (cm3/g) | Density (g/cm3) |
|---|---|---|---|---|
| 1.5-FDN-CNF aerogel | 12.63 | 106.25 | 0.8556 | 0.0363 |
| 2.5-FDN-CNF aerogel | 8.56 | 162.92 | 0.6812 | 0.0502 |
| 3.5-FDN-CNF aerogel | 7.78 | 175.85 | 0.6324 | 0.0542 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhu, W.; Huang, R.; Zhang, Y.; Jia, C.; Zhao, H.; Chen, W.; Xue, Y. Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques. Polymers 2020, 12, 2583. https://doi.org/10.3390/polym12112583
Wang Z, Zhu W, Huang R, Zhang Y, Jia C, Zhao H, Chen W, Xue Y. Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques. Polymers. 2020; 12(11):2583. https://doi.org/10.3390/polym12112583
Chicago/Turabian StyleWang, Zhe, Wenkai Zhu, Runzhou Huang, Yang Zhang, Chong Jia, Hua Zhao, Wei Chen, and Yuanyuan Xue. 2020. "Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques" Polymers 12, no. 11: 2583. https://doi.org/10.3390/polym12112583
APA StyleWang, Z., Zhu, W., Huang, R., Zhang, Y., Jia, C., Zhao, H., Chen, W., & Xue, Y. (2020). Fabrication and Characterization of Cellulose Nanofiber Aerogels Prepared via Two Different Drying Techniques. Polymers, 12(11), 2583. https://doi.org/10.3390/polym12112583