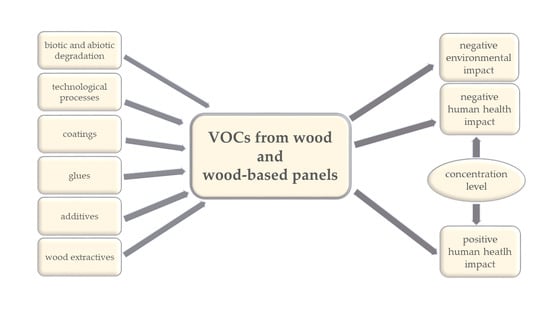
Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation
Abstract
:1. Introduction
2. VOCs from Wood
3. VOCs from Wood-Based Panels and Products
4. VOCs from Wood and Wood-Based Panels as a Potential Health Risk, Ways of their Mitigation
5. Analytical Methods to Assess VOCs
5.1. GC-MS for VOCs Detection from Wood and Wood-Based Panels
5.2. VOCs Extraction Techniques and Sample Introduction
5.2.1. Liquid Extractions from a Solid Sample
5.2.2. VOCs Sampling from Air
Headspace
Sorption Techniques Coupled to Thermal Desorption
SPME
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demirel, G.; Özden, Ö.; Dögerogly, T.; Gaga, E.O. Personal exposure of primary school children to BTEX, NO2 and ozone in Eskisehir, Turkey: Relationship with indoor/outdoor concentrations and risk assessment. Sci. Total Environ. 2014, 473–474, 537–548. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 16000-5. Indoor Air—Part 5: Sampling Strategy for Volatile Organic Compounds (VOCs); ISO/TC 146; Technical Committee: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Indoor Air Quality: Organic Pollutants; WHO Regional Office for Europe: Copenhagen, Denmark, 1989. [Google Scholar]
- Thurston, G.D. Outdoor Air Pollution: Sources, Atmospheric Transport, and Human Health Effects. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Elsevier: Cambridge, MA, USA, 2017; Volume 5, pp. 367–377. [Google Scholar]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Li, G.H.; Wei, W.; Shao, X.; Nie, L.; Wang, H.L. A comprehensive classification method for VOCs emission sources to tackle air pollution based on VOCs species reactivity and emission amounts. J. Environ. Sci. 2018, 67, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Dumergues, L.; Ponche, J.-L.; Torres, L. The biogenic volatile organic compounds emission inventory in France. Application to plant ecosystems in Berre-Marseilles area (France). Sci. Total Environ. 2006, 372, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Tie, X.X.; Li, G.H.; Ying, Z.M. Biogenic emissions of isoprenoids and NO in China and comparison to anthropogenic emissions. Sci. Total Environ. 2006, 371, 238–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raysoni, A.U.; Stock, T.H.; Sarnat, J.A.; Montoya Sosa, T.; Sarnat, S.E.; Holguin, F.; Greenwald, R.; Johnson, B.; Li, W.W. Characterization of traffic-related air pollutant metrics at four schools in El Paso, Texas, USA: Implications for exposure assessment and siting schools in urban areas. Atmos. Environ. 2013, 80, 140–151. [Google Scholar] [CrossRef]
- Hussein, T.; Paasonen, P.; Kulmala, M. Activity pattern of a selected group of school occupants and their family members in Helsinki—Finland. Sci. Total Environ. 2012, 425, 289–292. [Google Scholar] [CrossRef]
- Picot, S.D.; Watson, J.J.; Jones, J.W. A global inventory of volatile organic compound emissions from anthropogenic sources. J. Geophys. Res. 1992, 97, 9897–9912. [Google Scholar] [CrossRef]
- You, Z.Q.; Zhu, Y.; Jang, C.; Wang, S.X.; Gao, J.; Lin, C.H.J.; Li, M.; Zhu, Z.; Wei, H.; Yang, W. Response surface modeling-based source contribution analysis and VOCs emission control policy assessment in a typical ozone-polluted urban Shunde, China. J. Environ. Sci. 2017, 51, 294–304. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Formaldehyde, 2-butoxyethanol and 1-tertbutoxypropan-2-ol. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 88, 1–478. [Google Scholar]
- Billionnet, C.; Gay, E.; Kirchner, S.; Leynaert, B.; Annesi-Maesano, I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011, 111, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchi, T. Head Space—Solid Phase Micro Extraction Profile of Volatile Organic Compounds Emitted from Parquet Samples. J. Wood Chem. Technol. 2014, 34, 211–224. [Google Scholar] [CrossRef]
- Tanaka-Kagawa, T.; Uchiyama, S.; Matsushima, E.; Sasaki, A.; Kobayashi, H.; Kobayashi, H.; Yagi, M.; Tsuno, M.; Arao, M.; Ikemoto, K.; et al. Survey of volatile organic compounds found in indoor and outdoor air samples from Japan. Bull. Natl. Inst. Health Sci. 2005, 123, 27–31. [Google Scholar]
- Joshi, S.M. The sick building syndrome. Indian J. Occup. Environ. Med. 2008, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Maskell, D.; da Silva, C.F.; Mower, K.; Cheta, R.; Dengel, A.; Ball, R.; Ansell, M.; Walker, P.; Shea, A. Properties of bio-based insulation materials and their potential impact on indoor air quality. In Proceedings of the First International Conference on Bio-Based Building Materials, Clermont-Ferrand, France, 22–24 June 2015. [Google Scholar]
- Singleton, R.; Salkoski, A.J.; Bulkow, L.; Fish, C.; Dobson, J.; Albertson, L. Housing characteristics and indoor air quality in households of Alaska Native children with chronic lung conditions. Indoor Air 2017, 27, 478–486. [Google Scholar] [CrossRef]
- Jain, R.B. Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of U.S. adults. Environ. Toxicol. Pharmacol. 2015, 40, 471–479. [Google Scholar] [CrossRef]
- Cakmak, S.; Dales, R.E.; Liu, L.; Kauri, L.M.; Lemieux, C.H.L.; Hebbern, C.H.; Zhu, J. Residential exposure to volatile organic compounds and lung function: Results from a population-based cross-sectional survey. Environ. Pollut. 2014, 194, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Gminski, R.; Marutzky, R.; Kevekordes, S.; Fuhrmann, F.; Bürger, W.; Hauschke, D.; Ebner, W.; Mersch-Sundermann, V. Chemosensory irritations and pulmonary effects of acute exposure to emissions from oriented strand board. Hum. Exp. Toxicol. 2011, 30, 1204–1221. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-A.; An, J.-Y.; Kim, H.-J.; Kim, S.D.; Park, J.C. TVOC and formaldehyde emission behaviors from flooring materials bonded with environmental-friendly MF/PVAc hybrid resins. Indoor Air 2007, 17, 404–415. [Google Scholar] [CrossRef]
- Madureira, J.; Paciencia, I.; Pereira, C.; Teixeira, J.P.; Fernandes, E.O. Indoor air quality in Portuguese schools: Levels and sources of pollutants. Indoor Air 2016, 26, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.K. Occurence of volatile organic compounds in indoor air. In Organic Indoor Air Pollutants: Occurence and Measurement and Evaluation, 1st ed.; Salthammer, T., Ed.; Wiley-VCH: Weinheim, Germany, 1999; pp. 171–184. [Google Scholar]
- Van der Wal, J.F.; Hoogeveen, A.W.; Wouda, P. The Influence of Temperature on the Emission of Volatile Organic Compounds from PVC flooring, Carpet, and Paint. Indoor Air 1997, 7, 215–221. [Google Scholar] [CrossRef]
- Kirkeskov, L.; Witterseh, T.; Funch, L.W.; Kristiansen, E.; Mølhave, L.; Hansen, M.K.; Knudsen, B.B. Health evaluation of volatile organic compound (VOC) emission from exotic wood products. Indoor Air 2009, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Wiglusz, R.; Nikel, G.; Igielska, B.; Sitko, E. Volatile Organic Compounds Emissions from Particleboard Veneered with Decorative Paper Foil. Holzforschung 2002, 56, 108–110. [Google Scholar] [CrossRef]
- Ewen, R.J.; Jones, P.R.H.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N. Identification by gas chromatography-mass spectrometry of the volatile organic compounds emitted from the wood-rotting fungi Serpula lacrymans and Coniophora puteana, and from Pinus sylvestris timber. Mycol. Res. 2004, 108, 806–814. [Google Scholar] [CrossRef] [PubMed]
- An, J.-Y.; Kim, S.; Kim, H.-J. Formaldehyde and TVOC emission behavior of laminate flooring by structure of laminate flooring and heating condition. J. Hazard. Mater. 2011, 187, 44–51. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Indoor Air Quality: Dampness and Mould; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Koivula, M.; Kymäläinen, H.-R.; Virta, J.; Hakkarainen, H.; Hussein, T.; Komulainen, J.; Koponen, H.; Hautala, M.; Hämeri, K.; Kanerva, P.; et al. Emissions from thermal insulations—part 2: Evaluation of emissions from organic and inorganic insulations. Build. Environ. 2005, 40, 803–814. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice, 1st ed.; Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Gérard, J.; Paradis, S.; Thibaut, B. Survey on the chemical composition of several tropical wood species. Bois For. Trop. 2019, 342, 79–91. [Google Scholar] [CrossRef]
- Willför, S.; Hafizoglu, H.; Tümen, I.; Yazici, H.; Arfan, M.; Ali, M.; Hombom, B. Extractives of Turkish and Pakistani Tree Species. Holz Roh-Werkst. 2007, 65, 215–221. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and other Processes, 1st ed.; Wiley-Blackwell: Chichester, UK, 2006; pp. 25–30. [Google Scholar]
- Gierlinger, N.; Jacques, D.; Wimmer, R.; Pâques, L.E.; Schwanninger, M. Heartwood extractives and lignin content of different larch species (Larix sp.) and relationships to brown-rot decay-resistance. Trees Struct. Funct. 2004, 18, 230–236. [Google Scholar] [CrossRef]
- Liu, R.; Wang, C.; Huang, A.; Lv, B. Characterization of Odors of Wood by Gas Chromatography-Olfactometry with Removal of Extractives as Attempt to Control Indoor Air Quality. Molecules 2018, 23, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hse, C.-Y.; Kuo, M.-L. Influence of extractives on wood gluing and finishing—A review. For. Prod. J. 1988, 38, 52–56. [Google Scholar]
- Benouadah, N.; Pranovich, A.; Aliouche, D.; Hemming, J.; Smeds, A.; Willför, S. Analysis of extractives from Pinus halepensis and Eucalyptus camaldulensis as predominant trees in Algeria. Holzforschung 2018, 72, 97–104. [Google Scholar] [CrossRef]
- Hafizoglu, H.; Holmbom, B. Chemical composition of extractives from Abies nordmanniana. Holz Roh-Werkst. 1995, 53, 273–275. [Google Scholar] [CrossRef]
- Bajer, T.; Šulc, J.; Ventura, K.; Bajerová, P. Volatile compounds fingerprinting of larch tree samples for Siberian and European larch distinction. Eur. J. Wood Wood Prod. 2020, 78, 393–402. [Google Scholar] [CrossRef]
- Roffael, E. Volatile organic compounds and formaldehyde in nature, wood and wood based panels. Holz Roh-Werkst. 2006, 64, 144–149. [Google Scholar] [CrossRef]
- Back, E.L. Pattern of parenchyma and canal resin composition in softwoods and hardwoods. J. Wood Sci. 2002, 48, 167–170. [Google Scholar] [CrossRef]
- Gandelová, L.; Horáček, R.; Šlezingerová, J. Nauka o dřevě, 1st ed.; Mendel University: Brno, Czech Republic, 2002; ISBN 80-7157-577-1. [Google Scholar]
- Hiramatsu, Y.; Shida, S.; Miyazaki, Y. House dust mites and their sensitivity to wood oils and volatiles. J. Wood Sci. 2008, 54, 1–9. [Google Scholar] [CrossRef]
- Pei, J.; Yin, Y.; Tianjin, J.L. Long-term indoor gas pollutant monitor of new dormitories with natural ventilation. Energy Build. 2016, 129, 514–523. [Google Scholar] [CrossRef]
- Xi, Z.; Zhiwei, L.; Wu, Y. Human physiological responses to wooden indoor environment. Physiol. Behav. 2017, 174, 27–34. [Google Scholar]
- Kadir, R.; Hale, M.D. Antioxidant potential and content of phenolic compounds in extracts of twelve selected Malaysian commercial wood species. Eur. J. Wood Prod. 2017, 75, 615–622. [Google Scholar] [CrossRef]
- Nascimento, E.; Morais, S.; García-Vallejo, M. The Composition of Wood Extracts from Spanish Pinus Pinaster And Brazilian Pinus Caribaea. J. Braz. Chem. Soc. 1995, 6, 331–336. [Google Scholar] [CrossRef]
- Granström, K. Emissions of Volatile Organic Compounds from Wood. Ph.D. Thesis, Karlstad University, Karlstad, Sweden, 2005. [Google Scholar]
- Stachowiak-Wencek, A.; Pradzyński, W. Emission of volatile organic compounds from wood of exotic species. For. Wood Technol. 2014, 86, 215–219. [Google Scholar]
- Zimmer, K.; Melcher, E. A screening study on extractive content and composition of Scots pine heartwood of three stands with close proximity and their resistance against basidiomycetes. Int. Wood Prod. J. 2017, 8, 45–49. [Google Scholar] [CrossRef]
- Taylor, A.M.; Labbé, N.; Noehmer, A. NIR-based prediction of extractives in American white oak heartwood. Holzforschung 2011, 65, 185–190. [Google Scholar] [CrossRef]
- Tümen, I.; Reunanen, M. A Comparative Study on Turpentine Oils of Oleoresins of Pinus sylvestris L. from Three Districts of Denizli. Rec. Nat. Prod. 2010, 4, 224–229. [Google Scholar]
- Ioannidis, K.; Melliou, E.; Magiatis, P. High-Throughput 1H-Nuclear Magnetic Resonance-Based Screening for the Identification and Quantification of Heartwood Diterpenic Acids in Four Black Pine (Pinus nigra Arn.) Marginal Provenances in Greece. Molecules 2019, 24, 3603. [Google Scholar] [CrossRef] [Green Version]
- Krutul, D.; Zielenkiewicz, T.; Zawadzki, J.; Radomski, A.; Antczak, A.; Drożdżek, M. Influence of urban environment originated heavy metal pollution on the extractives and mineral substances content in bark and wood of oak (Queecus robur L.). Wood Res. 2014, 59, 177–190. [Google Scholar]
- Viiri, H.; Annila, E.; Kitunen, V.; Niemelä, P. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees 2001, 15, 112–122. [Google Scholar] [CrossRef]
- Englund, F.; Nussbaum, R.M. Monoterpenes in Scots Pine and Norway Spruce and their Emission during Kiln Drying. Holzforschung 2000, 54, 449–456. [Google Scholar] [CrossRef]
- Kačík, F.; Vel’ková, V.; Šmíra, P.; Nasswettrová, A.; Kačíková, D.; Reinprecht, L. Release of Terpenes from Fir Wood during Its Long-Term Use and in Thermal Treatment. Molecules 2012, 17, 9990–9999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, A.; Niemz, P. Extractives in some tropical woods. Eur. J. Wood Prod. 2012, 70, 79–83. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Characterisation of volatile organic compounds in stemwood using solid-phase microextraction. Phytochem. Anal. 2006, 17, 91–101. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Singh, A.; Raymond, L.; Hill, S.; Schmitt, U. Extractive distribution in Pseudotsuga menziesii: Effects on cell wall porosity in sapwood and heartwood. IAWA J. 2019, 40, 721–740. [Google Scholar] [CrossRef]
- Benouadah, N.; Aliouche, D.; Pranovich, A.; Willför, S. Chemical characterization of Pinus halepensis sapwood and heartwood. Wood Mater. Sci. Eng. 2019, 14, 157–164. [Google Scholar] [CrossRef]
- Forsthuber, B.; Ecker, M.; Truskaller, M.; Grüll, G. Rapid prediction of surface characteristics of European and Siberian larch wood by FT-NIRS. Eur. J. Wood Prod. 2016, 75, 569–580. [Google Scholar] [CrossRef]
- Eichhorn, S.; Erfurt, S.; Hofmann, T.; Seegmüller, S.; Németh, R.; Hapla, F. Determination of the phenolic extractive content in sweet chestnut (Castanea Sativa Mill.) Wood. Wood Res. 2017, 62, 181–196. [Google Scholar]
- Zahri, S.; Moubarik, A.; Charrier-El Bouhtoury, F.; Chaix, G.; Baillères, H.; Nepveu, G.; Charrier, B. Quantitative assessment of total phenol contents of European oak (Quercus petraea and Quercus robur) by diffuse reflectance NIR spectroscopy on solid wood surfaces. Holzsforschung 2008, 62, 679–687. [Google Scholar] [CrossRef]
- Dix, B.; Roffael, E.; Schneider, T. Abgabe von Flüchtigen Verbindungen (Volatile Organic Compounds, VOC) von Strands, Hergestellt aus Kernund Splintholz der Kiefer; Report 6; Wilhelm-Klauditz-Institut: Braunschweig, Germany, 2004. [Google Scholar]
- Sivrikaya, H.; Tesařová, D.; Jeřábková, E.; Can, A. Color change and emission of volatile organic compounds from Scots pine exposed to heat and vacuum-heat treatment. J. Build. Eng. 2019, 26, 100918. [Google Scholar] [CrossRef]
- Hyttinen, M.; Masalin-Weijo, M.; Kalliokoski, P.; Pasanen, P. Comparison of VOC emissions between air-dried and heat-treated Norway spruce (Picea abies), Scots pine (Pinus sylvesteris) and European aspen (Populus tremula) wood. Atmos. Environ. 2010, 44, 5028–5033. [Google Scholar] [CrossRef]
- Czajka, M.; Fabisiak, B.; Fabisiak, E. Emission of Volatile Organic Compounds from Heartwood and Sapwood of Selected Coniferous Species. Forests 2020, 11, 92. [Google Scholar] [CrossRef] [Green Version]
- Makowski, M.; Ohlmeyer, M. Comparison of a small and a large environmental test chamber for measuring VOC emissions from OSB made of Scots pine (Pinus sylvestris L.). Holz Roh-Werkst. 2006, 64, 469–472. [Google Scholar] [CrossRef]
- Manninen, A.-M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Wajs, A.; Pranovich, A.; Reunanen, M.; Willför, S.; Holmbom, B. Headspace-SPME analysis of the sapwood and heartwood of Picea Abies, Pinus Sylvestris and Larix Decidua. J. Essent. Oil Res. 2007, 19, 125–133. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Zhu, X.D. Headspace solid-phase microextraction for the determination of volatile organic compounds in Larix gmelini particles. Phys. Procedia 2012, 32, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Sutton, B.A.; Woosley, R.S.; David, J.; Butcher, D.J. Determination of monoterpenes in oleoresin: A chemosystematic study of the interaction between fraser fir (Abies fraseri) and balsam woolly adelgid (Adelges piceae). Microchem. J. 1997, 56, 332–342. [Google Scholar] [CrossRef]
- Baumann, M.G.D.; Batterman, S.A.; Zhang, G.-Z. Terpene emissions from particleboard and medium-density fiberboard products. Forest Prod. J. 1999, 49, 49–56. [Google Scholar]
- Sun, S.; Zhao, Z.; Shen, J. Effects of the manufacturing conditions on the VOCs emissions of particleboard. BioResources 2020, 15, 1074–1084. [Google Scholar] [CrossRef]
- Schäfer, M.; Roffael, E. On the formaldehyde release of wood. Holz Roh-Werkst. 2000, 58, 259–264. [Google Scholar] [CrossRef]
- Böhm, M.; Salem, M.Z.; Srba, J. Formaldehyde emission monitoring from a variety of solid wood, plywood, blockboard and flooring products manufactured for building and furnishing materials. J. Hazard. Mater. 2012, 221, 68–79. [Google Scholar] [CrossRef]
- Roffael, E.; Gabriel, M.; Schneider, T.; Behn, C. Influence of pulping technology on the release of formaldehyde and volatile organic acids from oak fibres and medium density fibreboards (MDF) prepared therefrom. Eur. J. Wood Prod. 2018, 76, 397–399. [Google Scholar] [CrossRef]
- Risholm-Sundman, M.; Lundgren, M.; Vestin, E.; Herder, P. Emission of acetic acid and other volatile organic compounds from different species of solid wood. Holz Roh Werkst. 1998, 56, 125–129. [Google Scholar] [CrossRef]
- Packman, D.F. The acidity of wood. Holzforschung 1960, 14, 178–183. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC emissions at different manufacturing stages of wood based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Masson, G.; Puech, J.L.; Moutonnet, M. Composition chimique du bois de chêne de tonnellerie. Bull. OIV. 1996, 69, 635–657. [Google Scholar]
- Hodgson, A.T.; Beal, D.; McIlvaine, J.E.R. Sources of formaldehyde, other aldehydes and terpenes in a new manufactured house. Indoor Air 2002, 12, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shen, J.; Zhu, X. Influence of processing parameters on VOC emission from particleboards. Environ. Monit. Assess. 2010, 171, 249–254. [Google Scholar] [CrossRef]
- Irle, M.; Barbu, M.C. Wood based Panel Technology. In Wood Based Panels: An Introduction for Specialists, 1st ed.; Thoemen, H., Irle, M., Sernek, M., Eds.; Brunel University Press: London, UK, 2010; pp. 1–55. [Google Scholar]
- Tong, R.; Zhang, L.; Yang, X.; Liu, J.; Zhou, P.; Li, J. Emission characteristics and probabilistic health risk of volatile organic compounds from solvents in wooden furniture manufacturing. J. Clean Prod. 2018, 208, 1096–1108. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Sources and Factors Affecting Indoor Air Emissions from Engineered Wood Products: Summary and Evaluation of Current Literature. Available online: https://www.smithgardnerinc.com/wp-content/uploads/2018/05/1996_Sources-and-Factors-Affecting-Indoor-Emissions-from-Engineered-Wood-Products-Summary-and-Evaluation-of-Current-Literature.pdf (accessed on 16 June 2020).
- Qi, Y.; Shen, L.; Zhang, J.; Yao, J.; Lu, R.; Miyakoshi, T. Species and release characteristics of VOCs in furniture coating process. Environ. Pollut. 2019, 245, 810–819. [Google Scholar] [CrossRef]
- Residential Energy Efficiency Database, Residential Indoor Air Quality; Information Technology Specialists Inc.: Bowie, MD, USA, 1996.
- Notheim, C.M.; Leovic, K.W.; Shaver, E.M. The Application of Pollution Prevention to Reduce Indoor Air Emissions from Office Equipment and From Composite Wood Materials; Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Grand View Research. Low-Volatile Organic Compounds Coating Additives Market Size, Share & Trends Analysis Report by Application, Regional Outlook, And Segment Forecasts, 2019 To 2025. Available online: https://www.grandviewresearch.com/industry-analysis/coating-additives-market (accessed on 16 June 2020).
- Johansson, I.; Karlsson, T.; Wimmerstedt, R. Volatile organic compound emissions when drying wood particles at high dewpoints. Chin. J. Chem. Eng. 2004, 12, 767–772. [Google Scholar]
- Gross, A.; Mocho, P.; Plaisance, H.; Cantau, C.; Kinadjian, N.; Yrieix, C.; Desauziers, V. Assessment of VOCs material/air exchanges of building products using the DOSEC®-SPME method. Energy Procedia 2017, 122, 367–372. [Google Scholar] [CrossRef]
- Su, W.; Hui, Y.; Banerjee, S. Field-proven strategies for reducing volatile organic carbons from hardwood drying. Environ. Sci. Technol. 1999, 33, 1056–1059. [Google Scholar] [CrossRef]
- Svedberg, U.R.A.; Högberg, H.-E.; Högberg, J.; Galle, B. Emission of hexanal and carbonmonoxide from storage of wood pellets, a potential occupational and domestic health hazard. Ann. Occup. Hyg. 2004, 48, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, O.; Brozowski, F.; Wiegner, K.; Braue, F. Bestimmung der VOC-Emissionen aus Grobspanplatten (OSB Platten) und ihre Bewertung nach dem AgBB-Schema. Umw. Mensch Inf. 2013, 1, 5–11. [Google Scholar]
- Fries, N. Nonanal as a growth factor for wood-rotting fungi. Nature 1960, 187, 166–167. [Google Scholar] [CrossRef]
- Spengler, J.D.; Samet, J.M.; McCarthy, J.F. Indoor Air Quality Handbook, 1st ed.; McGraw-Hill Professional: New York, NY, USA, 2001. [Google Scholar]
- Weisel, C.P.; Zhang, J.; Turpin, B.; Morandi, M.; Colome, S.; Stock, T.; Spektor, D.; Korn, L.; Winer, A.; Knon, J. Relationships of Indoor, Outdoor and Personal Air (RIOPA). Part. I. Collection Methods and Descriptive Analyses. Res. Rep. Health Eff. Inst. 2005, 130, 1–107. [Google Scholar]
- Däumling, C.; Wilke, O.; Horn, W.; Brenske, K.R.; Jann, O. Committee for Health-related Evaluation of Building Products. Health-related Evaluation Procedure for Volatile Organic Compounds Emissions (VOC and SVOC) from Building Products—A Contribution to the European Construction Products Directive. Gefahrst. Reinhalt. Luft 2005, 65, 90–92. [Google Scholar]
- Mølhave, L. Volatile Organic Compounds, Indoor Air Quality and Health. Indoor Air 1991, 1, 357–376. [Google Scholar] [CrossRef]
- Gminski, R.; Marutzky, R.; Kevekordes, S.; Fuhrmann, F.; Bürger, W.; Hauschke, D.; Ebner, W.; Mersch-Sundermann, V. Can VOC emissions from pinewood and oriented strand boards (OSB) affect human health? A controlled human exposure study. In Proceedings of the 10th International Conference on Healthy Buildings, Brisbane, Australia, 8–12 July 2012; Curran: Red Hook, NY, USA, 2013; Volume 1, pp. 278–279. [Google Scholar]
- Institute of Health Technology and Prevention Research. Stone Pine—Positive Health Effects of Stone Pine Furniture. Available online: http://www.zirbe.info/files/pdf_zirbenholz_folder_en.pdf (accessed on 26 June 2020).
- McDonald, A.G.; Wastney, S. Analysis of Volatile Emissions from Kiln Drying of Radiata Pine. In Proceedings of the 8th International Symposium on Wood and Pulping Chemistry, Helsinki, Finland, 6–9 June 1995; pp. 434–436. [Google Scholar]
- Jiang, C.; Li, D.; Zhang, P.; Li, J.; Wuang, J.; Yu, J. Formaldehyde and volatile organic compound (VOC) emissions from particleboard: Identification of odorous compounds and effects of heat treatment. Build. Environ. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Kim, S. The reduction of formaldehyde and VOCs emission from wood based flooring by green adhesive using cashew nut shell liquid (CNSL). J. Hazard. Mater. 2010, 182, 919–922. [Google Scholar] [CrossRef]
- Kim, S. The reduction of indoor air pollutant from wood based composite by adding pozzolan for building materials. Constr. Build. Mater. 2009, 23, 2319–2323. [Google Scholar] [CrossRef]
- Da Silva, C.F.; Stefanowski, B.; Maskell, D.; Ormondroyd, G.A.; Ansell, M.P.; Dengel, A.C.; Ball, R.J. Improvement of indoor air quality by MDF panels containing walnut shells. Build. Environ. 2017, 123, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Uitterhaegen, E.; Quang, H.N.; Othmane, M.; Stevens, C.H.V.; Talou, T.; Rigal, L.; Evon, P. New renewable and biodegradable fiberboards from a coriander press cake. J. Renew. Mater. 2016, 3, 225–238. [Google Scholar] [CrossRef]
- Simon, V.; Uitterhaegen, E.; Robillard, A.; Ballas, S.; Véronèse, T.; Vilarem, G.; Merah, O.; Talou, T.; Evon, P. VOC and carbonyl compound emissions of a fiberboard resulting from a coriander biorefinery: Comparison with two commercial wood-based building materials. Environ. Sci. Pollut. Res. 2020, 27, 16121–16133. [Google Scholar] [CrossRef] [PubMed]
- Adamová, T.; Hradecký, J.; Prajer, M. VOC Emissions from Spruce Strands and Hemp Shive: In Search for a Low Emission Raw Material for Bio-Based Construction Materials. Materials 2019, 12, 2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, X. Measurement of formaldehyde and VOCs emissions from wood based panels with nanomaterial added melamine impregnated paper. Constr. Build. Mater. 2014, 66, 132–137. [Google Scholar] [CrossRef]
- Kilic, A.; Altuntas, E. Wood and bark volatile compounds of Laurus nobilis L. Holz Roh Werkst. 2006, 64, 317–320. [Google Scholar] [CrossRef]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef] [Green Version]
- Hübschmann, H.-J. Handbook of GC-MS—Fundamentals and Applications, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- ISO 16000-6:2011. Indoor Air—Part 6: Determination of Volatile Organic Compounds in Indoor and Test. Chambre Air by Active Sampling on Tenax TA® Sorbent, Thermal Desorption and Gas. Chromatography Using MS or MS-FID.; ISO/TC 146; Technical Committee: Geneva, Switzerland, 2011. [Google Scholar]
- Bouchonnet, S. Introduction to GC-MS Coupling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-7251-5. [Google Scholar]
- Štulík, K. Analytické Separační Metody, 1st ed.; Karolinum: Praha, Czech Republic, 2005. [Google Scholar]
- Shellie, R.; Marriott, P.; Cornwell, C. Characterization and Comparison of Tea Tree and Lavender Oils by Using Comprehensive Gas Chromatography. J. High. Resol. Chromatogr. 2000, 23, 554–560. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured: Carol Stream, IL, USA, 2007; ISBN 13 978-1-932633-21-4. [Google Scholar]
- Macchioni, F.; Cioni, P.L.; Flamini, G.; Morelli, I.; Maccioni, S.; Ansaldi, M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour Fragr. J. 2003, 18, 139–143. [Google Scholar] [CrossRef]
- Uçar, G.; Balaban, M.; Usta, M. Volatile needle and wood extracts of oriental spruce Picea orientalis (L.). Flavour Fragr. J. 2003, 18, 368–375. [Google Scholar] [CrossRef]
- Senila, L.R.; Miclean, M.; Senila, M.; Roman, M.; Roman, C. New analysis method of furfural obtained from wood applying an autohydrolysis pretreatment. Rom. Biotech. Lett. 2013, 18, 7947–7955. [Google Scholar]
- Candelier, K.; Dumarc, S.; Pétrissans, A.; Pétrissans, M.; Kamdem, P.; Gérardin, P. Thermodesorption coupled to GC–MS to characterize volatiles formation kinetic during wood thermodegradation. J. Anal. Appl. Pyrolysis 2013, 101, 96–102. [Google Scholar] [CrossRef]
- Bertaud, F.; Crampon, C.; Badens, E. Volatile terpene extraction of spruce, fir and maritime pine wood: Supercritical CO2 extraction compared to classical solvent extractions and steam distillation. Holzforschung 2017, 71, 667–673. [Google Scholar] [CrossRef]
- Pérez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Analysis of volatile components of oak wood by solvent extraction and direct thermal desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 1997, 778, 427–434. [Google Scholar] [CrossRef]
- Cetera, P.; Russo, D.; Milell, L.; Todaro, L. Thermo-treatment affects Quercus cerris L. wood properties and the antioxidant activity and chemical composition of its by-product extracts. Ind. Crops Prod. 2019, 130, 380–388. [Google Scholar] [CrossRef]
- Todaro, L.; Russo, D.; Cetera, P.; Milell, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crops Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Vichi, S.; Santini, C.; Natali, N.; Riponi, C.; López-Tamames, E.; Buxaderas, S. Volatile and semi-volatile components of oak wood chips analysed by Accelerated Solvent Extraction (ASE) coupled to gas chromatography-mass spectrometry (GC–MS). Food Chem. 2007, 102, 1260–1269. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Shen, J.; Shao, X. A comparison of accelerated solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction for analysis of terpenoids and sterols in tobacco. Anal. Bioanal. Chem. 2005, 383, 1003–1008. [Google Scholar] [CrossRef]
- Richter, B.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Accelerated solvent extraction: A technology for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Flores Péres, V.; Saffi, J.; Melecchi, M.I.S.; Abad, F.C.; de Assis Jacques, R.; Martinez, M.M.; Oliveira, E.C.; Caramão, E.B. Comparison of soxhlet, ultrasound-assisted and pressurized liquid extraction of terpenes, fatty acids and Vitamin E from Piper gaudichaudianum Kunth. J. Chromatogr. A 2006, 1105, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. 10—Extraction Techniques of Phenolic Compounds and Other Bioactive Compounds from Medicinal and Aromatic Plants. In Engineering Tools in the Beverage Industry, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 283–314. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; International Centre for Science and High Technology: Trieste, Italy, 2008. [Google Scholar]
- Mirošová, P. Stanovení Těkavých Látek ve Vybraných Typech Nefermentovaných Čajů Metodou GC-MS. Master’s Thesis, Univerzita Tomáše Bati ve Zlíně, Zlín, Czech Republic, 2012. [Google Scholar]
- Opekar, F. Základní analytická chemie, 2nd ed.; Karolinum: Praha, Czech Republic, 2010. [Google Scholar]
- ISO 16000-9:2006. Indoor Air—Part 9: Determination of the Emission of Volatile Organic Compounds from Building Products and Furnishing—Emission Test Chamber Method; Technical Committee: Geneva, Switzerland, 2006. [Google Scholar]
- Nicolle, J.; Desauziers, V.; Mocho, P. Solid phase microextraction sampling for a rapid and simple on-site evaluation of volatile organic compounds emitted from building materials. J. Chromatogr. A 2008, 1208, 10–15. [Google Scholar] [CrossRef]
- Özel, M.Z.; Gögüs, F.; Lewis, A.C. Composition of Eucalyptus camaldulensis Volatiles Using Direct Thermal Desorption Coupled with Comprehensive Two-Dimensional Gas Chromatography—Time-of-Flight-Mass Spectrometry. J. Chromatogr. Sci. 2008, 46, 157–161. [Google Scholar] [CrossRef]
- Souza-Silva, É.; Pawliszyn, J. Recent Advances in Solid-Phase Microextraction for Contaminant Analysis in Food Matrices. Compr. Anal. Chem. 2017, 76, 483–517. [Google Scholar] [CrossRef]
- Ligor, M.; Buszewski, B. The comparison of solid phase microextraction-GC and static headspace-GC for determination of solvent residues in vegetable oils. J. Sep. Sci. 2008, 31, 364–371. [Google Scholar] [CrossRef]

| Extractives/Group of VOCs | VOC | Pine * | Spruce * | Larch * | Fir | Douglas Fir | Aspen | Oak | Beech | Wood Species | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration in the Test Chamber (µg/m3) Sapwood/Heartwood | Concentration in the Test Chamber (µg/m3) Sapwood/Heartwood | Concentration in the Test Chamber (µg/m3) Sapwood/Heartwood | |||||||||||
| Terpenes | α-pinene | 3459/294 | [31,44,60,70,71,73,74] | 119/320 | [60,71,75] | 126/509 | [76] | [61,77] | [78] | Reference | |||
| β-pinene | 13/16 | [44,60,70,74] | −/74 | [60,71,75] | 4/14 | [61,77] | [78] | ||||||
| Camphene | 23/10 | [44,70,71,74] | <1/− | [71,75] | <1/6 | [76] | [61,77] | ||||||
| Δ3-carene | 108/40 | [31,44,60,70,71,74] | 63/45 | [71,75] | 17/16 | [76] | [61,77] | [78] | |||||
| Limonene | 5/<1 | [31,44,70,74] | 30/19 | [60,71,75] | 13/7 | [61,77] | [78] | ||||||
| Aldehydes | Benzaldehyde | <1/6 | [70,71,74] | <1/1 | [75] | 7/3 | [79] | [71] | |||||
| Decanal | 11/16 | [70,74] | − | [71,75] | 7/− | [71] | |||||||
| Furfural | / | [70,71,74] | / | [71] | / | [71] | |||||||
| Hexanal | 4/162 | [31,44,70,71,74] | −/17 | [71,75] | 8/24 | [76,79] | [71] | [16] | |||||
| Nonanal | 4/12 | [71,74] | 4/12 | [71,75] | 7/10 | [76,79] | [71] | ||||||
| Octanal | 1/7 | [70,74] | <1/− | 5/5 | [71] | ||||||||
| Pentanal | − | [70,71,74] | − | −/18 | [71] | ||||||||
| Formaldehyde | / | [80,81] | / | [80,81] | / | [81] | [81,82] | [81] | |||||
| Acids | Acetic acid | / | [31,71,74] | / | [71] | / | [76,79] | [71] | [16,83] | [83] | |||
| Material | Aim | Analytical Method | Sample Extraction and Introduction Technique | Capillary Column (Length × Internal Diameter; Film Thickness) | Ref. |
|---|---|---|---|---|---|
| Larix sibirica vs. Larix decidua | variability in VOCs composition, VOCs intensity | GC-FID, GC-MS | SMPE: DVB-CAR-PDMS—50:30 μm | SLB-5 (30 m × 0.25 mm; 0.25 μm) | [43] |
| Picea abies | variability in VOCs composition, methods comparison | GC-MS | SPME: DVB-CAR-PDMS—50:30 μm; CAR-PDMS—75 μm; CW-DVB—70 μm; PDMS-DVB—65 μm/dynamic HS/hydrodistillation | HP-5 (30 m × 0.32 mm; 0.25 μm) | [63] |
| Larix gmelinii | variability in VOCs composition, methods comparison | GC-MS | SPME: PDMS—100 μm/static headspace | TR-V1 (30 m × 0.25 mm; 1.4 μm) | [76] |
| Serpula lacrymans,Coniophora puteana and Pinus sylvestris | variability in VOCs composition, methods comparison | GC-MS | SPME: PDMS—100 μm; polyacrylate—85 μm, Tenax GR tubes | HP-1, HP-5, HP-Innowax (30 m × 0.25 mm; 0.25 µm) | [31] |
| unspecified wood biomass | furfural extraction and identification | GC-MS | autohydrolysis; SPME: DVB-CAR-PDMS; * | HP-5 MS (30 m × 0.25 mm; 0.25 μm) | [128] |
| wooden parquets | variability in VOCs composition | GC-MS | SPME: DVB-CAR-PDMS—50:30 μm | HP-5MS (30 m × 0.25 mm; 0.25 μm) | [16] |
| Abies alba vs. Fagus sylvatica | methods comparison due to VOCs | GC-MS | glass TD tube with glass wool and TD | DB-5 (30 m × 0.25 mm; 0.25 μm) | [129] |
| Larix gmelinii | TVOC and VOCs quantification (µg m−3) | GC-MS | glass desiccator (0.015 m3) and Tenax TA© tubes | TR-V1 (30 m × 0.25 mm; 1.4 μm) | [79] |
| Picea abies, Pinus sylvestris vs. Populus tremula | TVOC comparison | TCT-GC-MS | metal chamber (0.12 m3) and Tenax GR | HP-5MS (50 m × *; 0.5 μm) | [71] |
| Pinus sylvestris | variability in VOCs, quantification | GC-MS | FLEC (0.00035 m3) and Tenax TA© tubes | * | [70] |
| Pinus sylvestris | TVOC, relative proportion (% of total emission) of different compound groups and individual compounds | GC-MS | glass container (0.015 m3) and Tenax TA© tubes | HP-5 (50 m × 0.2 mm; 0.5 μm) | [74] |
| MDF | TVOC emission rate (mg m−2 h−1) | GC-MS | chamber (0.020 m3) and Tenax TA© tubes | RTX-1 (105 m × 0.32 mm; 3 µm) | [112] |
| PB and MDF from various tree kinds | VOCs quantification | GC-MS | stainless-steel chamber (0.053 m3) and cryotrap | EC-5 (30 m × 0.25 mm; 25 μm) | [78] |
| organic vs. unorganic insulation | TVOC | GC-MS | stainless-steel chamber (0.58 m3) and Tenax TA© tubes | fused silica column (25 m × 0.32 mm; *) | [33] |
| OSB from Pinus sylvestris | aldehydes and terpenes—chambers comparison | GC-MS | glass desiccator (0.023 m3) and stainless-steel chamber (1 m3) and Tenax TA© tubes, TDS 3 | * | [73] |
| OSB | individual VOCs quantification | GC-MS | glass desiccator and Tenax TA© tubes | * | [101] |
| Coatings in a furniture workshop | variability in VOCs composition, quantification | GC-MS | Tenax TA© tubes | DA-WAX (30 m × 0.25 m; 0.25 μm) | [93] |
| Pinus silvestris vs. Picea abies | abundance of monoterpenes | GC-MS | Tenax TA© tubes—acetone and Soxtec© | DB-Wax (30 m × 0.25 mm; 0.25 μm) | [60] |
| 12 various tropical wood species | total amount of extractives (% to dry wood) | GC-MS | sodium hydroxide and Soxhlet | HP-1 (25 m × 0.2 mm; 0.11 μm) | [62] |
| Populus cathayana vs. Hevea brasiliensis | individual VOCs% | GC-MS/O | ethanol and toluene and Soxhlet | DB-Wax (30 m × 0.25 mm; 0.25 μm) | [39] |
| Larix gmelinii PB | individual VOCs% | GC-MS | methylene chlorid and Soxhlet | * | [77] |
| Picea abies vs. Abies alba | individual VOCs quantification, methods comparison | GC-FID, GC-MS | ASE vs. steam distillation vs. Soxhlet | DB-5 (30 m × *; *) | [130] |
| Abies alba Mill. | VOCs reduction as protection from wood decay | GC-MS | extraction by hexane in Promax 2020 shaker | HP-5 MS (30 m × 0.25 mm; 0.25 μm) | [61] |
| Quercus alba, Quercus robur vs. Quercus pedunculata | specific VOCs quantification (cis- and trans-ß-methyl-γ-octalactone, eugenol, vanillin and syringaldehyde) | (DTD)-GC-MS | extraction by dichlormethane | SPB-1 (50 m × 0.2 mm; 0.25 μm) | [131] |
| Construction materials | VOCs emission from construction material | GC-(FID)-MS | DOSEC-SPME | * | [98] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamová, T.; Hradecký, J.; Pánek, M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers 2020, 12, 2289. https://doi.org/10.3390/polym12102289
Adamová T, Hradecký J, Pánek M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers. 2020; 12(10):2289. https://doi.org/10.3390/polym12102289
Chicago/Turabian StyleAdamová, Tereza, Jaromír Hradecký, and Miloš Pánek. 2020. "Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation" Polymers 12, no. 10: 2289. https://doi.org/10.3390/polym12102289
APA StyleAdamová, T., Hradecký, J., & Pánek, M. (2020). Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers, 12(10), 2289. https://doi.org/10.3390/polym12102289