Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
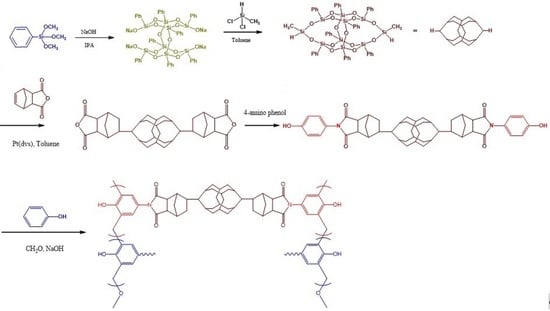
2.2. PDDSQ hybrids
2.3. Carbon/DDSQ Hybrids
3. Results and Discussion
3.1. Synthesis of the Pure PDDSQ Hybrid
3.2. Synthesis of PDDSQ Hybrids
3.3. Synthesis of Carbon/DDSQ Hybrids for Electrochemical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chee, S.S.; Jawaid, M. The Effect of Bi-Functionalized MMT on Morphology, Thermal Stability, Dynamic Mechanical, and Tensile Properties of Epoxy/Organoclay Nanocomposites. Polymers 2019, 11, 2012. [Google Scholar] [CrossRef] [Green Version]
- Ayat, M.; Rahmouni, A.; Belbachir, M.; Bensaada, N.; Baghdadli, M.C.; Meghabar, R. Thermoplastic block copolymer: Alpha-MethylStyrene and vinyl acetate catalyzed by clay layered called Maghnite-Na+ (Algerian MMT). J. Polym. Res. 2019, 26, 230. [Google Scholar] [CrossRef]
- He, J.; Xiao, P.; Liu, W.; Shi, J.; Zhang, L.; Liang, Y.; Pan, C.; Kuo, S.W.; Chen, T. A Universal high accuracy wearable pulse monitoring system via high sensitivity and large linearity graphene pressure sensor. Nano Energy 2019, 59, 422–433. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Sanchez, J.A.L.; Capilla, R.P.; Diaz, P.G. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Xiao, P.; Shi, J.; Liang, Y.; Lu, W.; Chen, Y.; Wang, W.; Theato, P.; Kuo, S.W.; Chen, T. High Performance Humidity Fluctuation Sensor for Wearable Devices via a Bioinspired Atomic-Precise Tunable Graphene-Polymer Heterogeneous Sensing Junction. Chem. Mater. 2018, 30, 4343–4354. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Silica and Carbon Nanocomposites Based on Polybenzoxazines. Macromol. Chem. Phys. 2019, 220, 1800306. [Google Scholar] [CrossRef]
- Kanimozhi, C.; Shea, M.J.; Ko, J.; Wei, W.; Huang, P.S.; Arnold, M.S.; Gopalan, P. Removable Nonconjugated Polymers To Debundle and Disperse Carbon Nanotubes. Macromolecules 2019, 52, 4278–4286. [Google Scholar] [CrossRef]
- Zhao, G.X.; Huang, X.B.; Tang, Z.W.; Huang, Q.F.; Niu, F.L.; Wang, X.K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Polybenzoxazine/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites. Polymers 2016, 8, 225. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2019, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.C.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, R.C.; Tseng, S.M.; Kuo, S.W. Minimizing the Strong Screening Effect of Polyhedral Oligomeric Silsesquioxane Nanoparticles in Hydrogen-Bonded Random Copolymers. Polymers 2018, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Shah, S.M.; Hussain, H. Amphiphilic tadpole-shaped POSS-poly(glycerol methacrylate) hybrid polymers: Synthesis and self-assembly. J. Polym. Res. 2019, 26, 4. [Google Scholar] [CrossRef]
- Byun, H.Y.; Choi, M.H.; Chung, J. Synthesis and characterization of resol type phenolic resin/layered silicate nanocomposites. Chem. Mater. 2001, 13, 4221–4226. [Google Scholar] [CrossRef]
- Ma, C.C.C.; Sung, S.C.; Wang, F.Y.; Chiang, L.Y.; Wang, L.Y.; Chiang, C.L. Thermal, mechanical, and morphological properties of novolac-type phenolic resin blended with fullerenol polyurethane and linear polyurethane. J. Polym. Sci. Polym. Phys. 2001, 39, 2436–2443. [Google Scholar] [CrossRef]
- Wei, J.; Wang, G.; Chen, F.; Bai, M.; Liang, Y.; Wang, H.; Zhao, D.; Zhao, Y. Sol–Gel Synthesis of Metal–Phenolic Coordination Spheres and Their Derived Carbon Composites. Angew Chem. Int. Ed. 2018, 130, 9986–9991. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Kuo, S.W.; Huang, W.J.; Lee, H.Y.; Chang, F.C. Miscibility, specific interactions, and self-assembly behavior of phenolic/polyhedral oligomeric silsesquioxane hybrids. J. Polym. Sci. Polym. Phys. 2004, 42, 1127–1136. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, H.C.; Huang, W.J.; Huang, C.F.; Chang, F.C. Hydrogen bonding interactions and miscibility between phenolic resin and octa (acetoxystyryl) polyhedral oligomeric silsesquioxane (AS-POSS) nanocomposites. J. Polym. Sci. Polym. Phys. 2006, 44, 673–686. [Google Scholar] [CrossRef]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Hirano, C.; Suzuki, Y.; Hayakawa, T.; Kuo, S.W. Strong Screening Effect of Polyhedral Oligomeric Silsesquioxanes (POSS) Nanoparticles on Hydrogen Bonded Polymer Blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef] [Green Version]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Maeda, R.; Hayakawa, T.; Kuo, S.W. Hydrogen Bond Interactions Mediate Hierarchical Self-Assembly of POSS-Containing Block Copolymers Blended with Phenolic Resin. Macromolecules 2014, 47, 8709–8721. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, S.W. Phenolic Functionality of Polyhedral Oligomeric Silsesquioxane Nanoparticles Affects Self-Assembly Supramolecular Structures of Block Copolymer Hybrid Complexes. Ind. Eng. Chem. Res. 2018, 57, 2546–2559. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, S.H.; Mitra, Y.; Kaiwen, L.; Pittman, U., Jr. Phenolic resin–trisilanolphenyl polyhedral oligomeric silsesquioxane POSS hybrid nanocomposites: Structure and properties. Polymer 2006, 47, 2984–2996. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zeng, K.; Zheng, S. Organic–inorganic hybrid nanocomposites involving novolac resin and polyhedral oligomeric Silsesquioxane. React. Funct. Polym. 2007, 67, 627–635. [Google Scholar] [CrossRef]
- Lei, Z.; Ji, J.; Wu, Q.; Zhang, J.; Wang, Y.; Jing, X.; Liu, Y. Curing behavior and microstructure of epoxy-POSS modified novolac phenolic resin with different substitution degree. Polymer 2019, 178, 121587. [Google Scholar] [CrossRef]
- Lin, H.C.; Kuo, S.W.; Huang, C.F.; Chang, F.C. Thermal and surface properties of phenolic nanocomposites containing octaphenol polyhedral oligomeric silsesquioxane. Macromol. Rapid Commun. 2006, 27, 537–541. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zheng, S. Organic–inorganic polyurethanes with 3,13-dihydroxypropyloctaphenyl double-decker silsesquioxane chain extender. Polym. Chem. 2013, 4, 1491–1501. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, K.; Wang, L.; Zheng, S. Poly(hydroxyl urethane)s with Double Decker Silsesquioxanes in the Main Chains: Synthesis, Shape Recovery, and Reprocessing Properties. Macromolecules 2020, 53, 434–444. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; Wang, L.; Zheng, S. Organic-inorganic polybenzoxazine copolymers with double decker silsesquioxanes in the main chains: Synthesis and thermally activated ring-opening polymerization behavior. Polymer 2017, 109, 254–265. [Google Scholar] [CrossRef]
- Liao, Y.T.; Lin, Y.C.; Kuo, S.W. Highly Thermally Stable, Transparent, and Flexible Polybenzoxazine Nanocomposites by Combination of Double-Decker-Shaped Polyhedral Silsesquioxanes and Polydimethylsiloxane. Macromolecules 2017, 50, 5739–5747. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W. Ortho-Imide and Allyl Groups Effect on Highly Thermally Stable Polybenzoxazine/Double-Decker-Shaped Polyhedral Silsesquioxane Hybrids. Macromolecules 2018, 51, 9602–9612. [Google Scholar] [CrossRef]
- Zhao, B.; Mei, H.; Liu, N.; Zheng, S. Organic–Inorganic Polycyclooctadienes with Double-Decker Silsesquioxanes in the Main Chains: Synthesis, Self-Healing, and Shape Memory Properties Regulated with Quadruple Hydrogen Bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
- Chen, W.C.; Tsao, Y.H.; Wang, C.F.; Huang, C.F.; Dai, L.; Chen, T.; Kuo, S.W. Main Chain–Type Block Copolymers through Atom Transfer Radical Polymerization from Double-Decker–Shaped Polyhedral Oligomeric Silsesquioxane Hybrids. Polymers 2020, 12, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Hayakawa, T.; Kikuchi, R.; Grunzinger, S.J.; Kakimoto, M.; Oikawa, H. Synthesis and characterization of semiaromatic polyimides containing POSS in main chain derived from double-decker-shaped silsesquioxane. Macromolecules 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kakimoto, M.; Oikawa, H. Synthesis and Characterization of Organosoluble Aromatic Polyimides Containing POSS in Main Chain Derived from Double Decker Shaped Silsesquioxane. Macromolecules 2008, 41, 3481–3487. [Google Scholar] [CrossRef]
- Chen, W.C.; Ahmed, M.M.M.; Wang, C.F.; Huang, C.F.; Kuo, S.W. Highly thermally stable mesoporous Poly (cyanate ester) featuring double-decker–shaped polyhedral silsesquioxane framework. Polymer 2019, 185, 121940. [Google Scholar] [CrossRef]
- Wang, C.F.; Ejeta, D.D.; Wu, J.Y.; Kuo, S.W.; Lin, C.H.; Lai, J.Y. Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions. Polymers 2020, 12, 523. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.Y.; Ma, C.C.M.; Wu, W.J. Kinetic Parameters of Thermal Degradation of Polyethylene Glycol-Toughened Novolac-Type Phenolic Resin. J. Appl. Polym. Sci. 2001, 80, 188–196. [Google Scholar] [CrossRef]
- Turri, S.; Levi, M. Structure, dynamic properties, and surface behavior of nanostructured ionomeric polyurethanes from reactive polyhedral oligomeric silsesquioxanes. Macromolecules 2005, 38, 5569–5574. [Google Scholar] [CrossRef]
- Turri, S.; Levi, M. Wettability of polyhedral oligomeric silsesquioxane nanostructured polymer surfaces. Macromol. Rapid Commun. 2005, 26, 1233–1236. [Google Scholar] [CrossRef]
- Mohamed, G.M.; Hung, W.S.; EL-Mahdy, A.F.M.; Ahmed, M.M.M.; Dai, L.; Chen, T.; Kuo, S.W. High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture. Polymers 2020, 12, 1193. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Liu, T.E.; Kuo, S.W. Direct synthesis of nitrogen-doped mesoporous carbons from triazine-functionalized resol for CO2 uptake and highly efficient removal of dyes. J. Hazard. Mater. 2020, 391, 122163. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Li, C.; Zhu, C.; Wang, S.; Zhang, X.; Chen, Y. Growth of MoSe2 nanosheets with small size and expanded spaces of (002) plane on the surfaces of porous N-doped carbon nanotubes for hydrogen production. Nanoscale 2016, 8, 16886–16893. [Google Scholar] [CrossRef] [PubMed]
- EL-Mahdy, A.F.M.; Kuo, C.H.; Alshehri, A.A.; Kim, J.; Young, C.; Yamauchi, Y.; Kuo, S.W. Strategic design of triphenylamine-and triphenyltriazine-based two-dimensional covalent organic frameworks for CO2 uptake and energy storage. J. Mater. Chem. A 2018, 6, 19532–19541. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.W. Hollow Microspherical and Microtubular [3+3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interfaces 2019, 11, 9343–9354. [Google Scholar] [CrossRef]
- Conway, B. Electrochemical Supercapacitors: Scientific Principles and Technological Application; Kluwer Academic/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; He, H.; Xu, L.; Zhao, K.; Zheng, C.; Zhang, C. A mesoporous conjugated polymer based on a high free radical density polytriphenylamine derivative: Its preparation and electrochemical performance as a cathode material for Li-ion batteries. J. Mater. Chem. A 2017, 5, 2701–2709. [Google Scholar] [CrossRef]
- Hu, F.; Wang, J.; Hu, S.; Li, L.; Shao, W.; Qiu, J.; Lei, Z.; Deng, W.; Jiang, X. Engineered Fabrication of Hierarchical Frameworks with Tuned Pore Structure and N,O-Co-Doping for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 31940–31949. [Google Scholar] [CrossRef]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Hung, Y.H.; Mansoure, T.H.; Yu, H.H.; Chen, T.; Kuo, S.W. A Hollow Microtubular Triazine-and Benzobisoxazole-Based Covalent Organic Framework Presenting Sponge-Like Shells That Functions as a High-Performance Supercapacitor. Chem. Asian J. 2019, 14, 1429–1435. [Google Scholar] [CrossRef]
- Hong, M.S.; Lee, S.H.; Kim, S.W. Use of KCl Aqueous Electrolyte for 2V Manganese Oxide/Activated Carbon Hybrid Capacitor. Electrochem. Solid-State Lett. 2002, 5, A227–A230. [Google Scholar] [CrossRef]
- Choi, B.G.; Chang, S.-J.; Kang, H.-W.; Park, C.P.; Kim, H.J.; Hong, W.H.; Lee, S.; Huh, Y.S. High Performance of a Solid-State Flexible Asymmetric Supercapacitor based on Graphene Films. Nanoscale 2012, 4, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Miao, M. Asymmetric Carbon Nanotube−MnO2 Two-Ply Yarn Supercapacitors for Wearable Electronics. Nanotechnology 2014, 25, 135401. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core–Shell Metal–Organic Frameworks: A New Method for Selectively. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.; Nayak, A.K.; Muhammad, R.; Pradhan, D.; Mohanty, P. Nitrogen-enriched nanoporous polytriazine for high-performance supercapacitor application. ACS Sustainable Chem. Eng. 2018, 6, 5895–5902. [Google Scholar] [CrossRef]
- Ai, W.; Zhou, W.; Du, Z.; Du, Y.; Zhang, H.; Jia, X.; Xie, L.; Yi, M.; Yu, T.; Huang, W. Benzoxazole and benzimidazole heterocycle-grafted graphene for high-performance supercapacitor electrodes. J. Mater. Chem. 2012, 22, 23439–23446. [Google Scholar] [CrossRef]
- Puthusseri, D.; Aravindan, V.; Mahdavi, S.; Ogale, S. 3D micro-porous conducting carbon beehive by single step polymer carbonization for high performance supercapacitors: The magic of in situ porogen formation. Energy. Environ. Sci. 2014, 7, 728–735. [Google Scholar] [CrossRef]
- Mohamed, G.M.; EL-Mahdy, A.F.M.; Takashi, Y.; Kuo, S.W. Ultrastable conductive microporous covalent triazine frameworks based on pyrene moieties provide high-performance CO2 uptake and supercapacitance. New J. Chem. 2020, 44, 8241–8253. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.W. Pyrene-functionalized tetraphenylethylene polybenzoxazine for dispersing single-walled carbon nanotubes and energy storage. Compos. Sci. Tech. 2020, 199, 108360. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Hung, Y.H.; Mansoure, T.H.; Yu, H.H.; Hsu, Y.S.; Wu, K.C.W.; Kuo, S.W. Synthesis of [3+3] bketoenamine-tethered covalent organic frameworks (COFs) for high-performance supercapacitance and CO2 storage. J. Taiwan Inst. Chem. Eng. 2019, 103, 199–208. [Google Scholar] [CrossRef]
- Laing, Z.; Liu, H.; Zeng, J.; Zhou, J.; Li, H.; Xia, H. Facile Synthesis of Nitrogen-Doped Microporous Carbon Spheres for High Performance Symmetric Supercapacitors. Nanoscale Res. Lett. 2018, 13, 314. [Google Scholar] [CrossRef] [Green Version]












| Name | Monomer Feed (g) | DDSQ-4OH in hybrids (wt.%) | Td (°C) | Char Yield (wt.%) | Mn (g/mol) | |||
|---|---|---|---|---|---|---|---|---|
| Phenol | DDSQ-4OH | CH2O | NaOH | FTIR | ||||
| Pure Phenolic | 10.0 | - | 17.25 | 4.25 | 0 | 364 | 41.6 | 5440 |
| PDDSQ-20 | 9.0 | 1.0 | 15.72 | 3.87 | 20.2 | 389 | 47.7 | 4670 |
| PDDSQ-30 | 4.0 | 1.0 | 7.09 | 1.75 | 32.3 | 403 | 49.7 | 4320 |
| PDDSQ-45 | 2.3 | 1.0 | 4.22 | 1.04 | 44.9 | 411 | 55.5 | 4580 |
| PDDSQ-50 | 1.5 | 1.0 | 2.78 | 0.68 | 50.7 | 438 | 58.2 | 4160 |
| PDDSQ-80 | 1.0 | 1.0 | 1.92 | 0.47 | 80.5 | 443 | 63.8 | 4460 |
| Pure PDDSQ | - | 1.0 | 0.19 | 0.04 | 100 | 532 | 70.4 | 4500 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Liu, Y.-T.; Kuo, S.-W. Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors. Polymers 2020, 12, 2151. https://doi.org/10.3390/polym12092151
Chen W-C, Liu Y-T, Kuo S-W. Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors. Polymers. 2020; 12(9):2151. https://doi.org/10.3390/polym12092151
Chicago/Turabian StyleChen, Wei-Cheng, Yuan-Tzu Liu, and Shiao-Wei Kuo. 2020. "Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors" Polymers 12, no. 9: 2151. https://doi.org/10.3390/polym12092151
APA StyleChen, W. -C., Liu, Y. -T., & Kuo, S. -W. (2020). Highly Thermal Stable Phenolic Resin Based on Double-Decker-Shaped POSS Nanocomposites for Supercapacitors. Polymers, 12(9), 2151. https://doi.org/10.3390/polym12092151