Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microbial Strains
2.3. Bacterial Cellulose Production and Purification
2.4. Preparation of BC Slurry
2.5. Yeast Biomass Production
2.6. Preparation of BC-Based Composites Films with CMC, Gly, and Yeast
2.7. Characterization
2.7.1. Solubility and Moisture Content
2.7.2. Mechanical Testing
2.7.3. Field-Emission Scanning Electron Microscopy
2.7.4. Fourier-Transform Infrared Spectroscopy
2.7.5. Thermogravimetric Analysis
2.8. Antibacterial Activity
2.9. Biocompatibility Evaluation
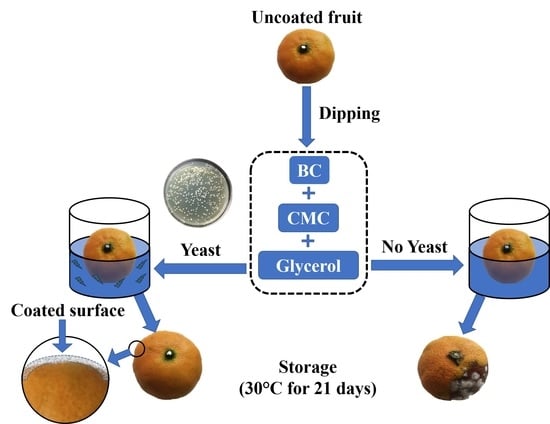
2.10. Fruit Packaging via Dipping Method
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation, Appearance, Moisture Content, and Water Solubility of BC/CMC/Gly/Yeast Composite Films
3.2. Mechanical Properties of BC/CMC/Gly/Yeast Composite Films
3.3. Morphology of BC/CMC/Gly/Yeast Composite Films
3.4. Chemical Properties of BC/CMC/Gly/Yeast Composite Films
3.5. Thermal Stability of BC/CMC/Gly/Yeast Composite Films
3.6. Antibacterial Activity of BC/CMC/Gly/Yeast Composite Films
3.7. Biocompatibility of BC/CMC/Gly/Yeast Composite Films
3.8. Real Packaging of Orange and Tomato with BC/CMC/Gly/Yeast Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira de Abreu, D.A.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Otto, S.; Strenger, M.; Maier-Nöth, A.; Schmid, M. Food packaging and sustainability—Consumer perception vs. correlated scientific facts: A review. J. Clean. Prod. 2021, 298, 126733. [Google Scholar] [CrossRef]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Han, J.H. Edible films and coatings: A review. Innov. Food Packag. 2014, 40, 213–255. [Google Scholar]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Effect of surface coatings on the shelf life and quality of cassava. J. Food Res. 2018, 7, 46–60. [Google Scholar]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Matharu, A.S.; de Melo, E.M.; Remón, J.; Wang, S.; Abdulina, A.; Kontturi, E. Processing of Citrus Nanostructured Cellulose: A Rigorous Design-of-Experiment Study of the Hydrothermal Microwave-Assisted Selective Scissoring Process. ChemSusChem 2018. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Xiong, Z.; Pan, L.; Luo, Q.; Xu, X.; Lu, S. Water-Induced shape memory effect of nanocellulose papers from sisal cellulose nanofibers with graphene oxide. Carbohydr. Polym. 2018. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr. Polym. 2020, 229, 115513. [Google Scholar] [CrossRef]
- Ullah, M.W.; Manan, S.; Kiprono, S.J.; Ul-Islam, M.; Yang, G. Synthesis, Structure, and Properties of Bacterial Cellulose. In Nanocellulose: From Fundamentals to Advanced Materials; Huang, J., Dufresne, A., Lin, N., Eds.; Wiley: Weinheim, Germany, 2019; pp. 81–113. [Google Scholar]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard Mater. 2018. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ullah, M.W.; Ul-Islam, M.; Khan, S.; Jang, J.H.; Park, J.K. Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 2019, 142, 135–144. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul Islam, M.; Khan, S.; Shah, N.; Park, J.K. Recent advancements in bioreactions of cellular and cell-free systems: A study of bacterial cellulose as a model. Korean J. Chem. Eng. 2017, 34, 1591–1599. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Mao, L.; Hu, S.; Gao, Y.; Wang, L.; Zhao, W.; Fu, L.; Cheng, H.; Xia, L.; Xie, S.; Ye, W.; et al. Biodegradable and Electroactive Regenerated Bacterial Cellulose/MXene (Ti3C2Tx) Composite Hydrogel as Wound Dressing for Accelerating Skin Wound Healing under Electrical Stimulation. Adv. Healthc. Mater. 2020, 9, 2000872. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jasim, A.; Zhao, W.; Fu, L.; Ullah, M.W.; Shi, Z.; Yang, G. Fabrication of pH-electroactive Bacterial Cellulose/Polyaniline Hydrogel for the Development of a Controlled Drug Release System. ES Mater. Manuf. 2018, 41–49. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Kamal, T.; Ul-Islam, S.; Shah, N.; Park, J.K. Recent advancement in cellulose based nanocomposite for addressing environmental challenges. Recent Pat. Nanotechnol. 2016, 10, 169–180. [Google Scholar] [CrossRef]
- Andrade, F.; Pertile, R.; Dourado, F.; Gama, F.M. Bacterial Cellulose: Properties, Production and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of bacterial cellulose from alternative cheap and waste resources: A step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Indrarti, L.; Indriyati Syampurwadi, A.; Pujiastuti, S. Physical and mechanical properties of modified bacterial cellulose composite films. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2016; Volume 1711, p. 050007. [Google Scholar]
- Chua, K.Y.; Azzahari, A.D.; Abouloula, C.N.; Sonsudin, F.; Shahabudin, N.; Yahya, R. Cellulose-based polymer electrolyte derived from waste coconut husk: Residual lignin as a natural plasticizer. J. Polym. Res. 2020, 27, 115. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Olsen, C.W.; Avena-Bustillos, R.J.; Friedman, M.; McHugh, T.H. Physical and antibacterial properties of edible films formulated with apple skin polyphenols. J. Food Sci. 2011, 76, M149–M155. [Google Scholar] [CrossRef]
- Ruas, F.A.D.; Guerra-Sá, R. In silico Prediction of Protein–Protein Interaction Network Induced by Manganese II in Meyerozyma guilliermondii. Front. Microbiol. 2020, 11, 236. [Google Scholar] [CrossRef] [Green Version]
- Bertini, E.V.; Leguina, A.C.; del Figueroa, L.I.C.; Nieto-Penalver, C.G. Endophytic microorganisms Agrobacterium tumefaciens 6N2 and Meyerozyma guilliermondii 6N serve as models for the study of microbial interactions in colony biofilms. Rev. Argent. Microbiol. 2019, 51, 286–287. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Di Cagno, R.; Trani, A.; Cardinali, G.; Gobbetti, M. Antifungal activity of Meyerozyma guilliermondii: Identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol. 2013, 33, 243–251. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of biological control agents and physical treatments in maintaining the quality of fresh and minimally-processed fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 60, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Zhang, Y.; Liu, J.; Cheng, Y.; Wang, J.; Pi, F.; Zhang, Y.; Sun, X. Perspective of Microbe-based Minerals Fortification in Nutrition Security. Food Rev. Int. 2020, 1–14. [Google Scholar] [CrossRef]
- Qadri, R.; Azam, M.; Khan, I.; Yang, Y.; Ejaz, S.; Akram, M.T.; Khan, M.A. Conventional and Modern Technologies for the Management of Post-Harvest Diseases. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Springer: Berlin/Heidelberg, Germany, 2020; pp. 137–172. [Google Scholar]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [Green Version]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of bioactive edible coatings loaded with Lactobacillus plantarum on physicochemical properties of fresh strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- Jasim, A.; Ullah, M.W.; Shi, Z.; Lin, X.; Yang, G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr. Polym. 2017, 163, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Sarlin, P.J.; Philip, R. Efficacy of marine yeasts and baker’s yeast as immunostimulants in Fenneropenaeus indicus: A comparative study. Aquaculture 2011, 321, 173–178. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Di, Z.; Shi, Z.; Ullah, M.W.; Li, S.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly (2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef]
- Tranoudis, I.; Efron, N. Tensile properties of soft contact lens materials. Contact Lens Anterior Eye 2004, 27, 177–191. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Younis, G.; Awad, A.; Dawod, R.E.; Yousef, N.E. Antimicrobial activity of yeasts against some pathogenic bacteria. Vet. World 2017, 10, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.; Picouet, P.; Lloret, E. Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. Int. J. Food Microbiol. 2010, 142, 222–228. [Google Scholar] [CrossRef]
- Conte, A.; Scrocco, C.; Brescia, I.; Del Nobile, M.A. Packaging strategies to prolong the shelf life of minimally processed lampascioni (Muscari comosum). J. Food Eng. 2009, 90, 199–206. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Scrocco, C.; Brescia, I. New strategies for minimally processed cactus pear packaging. Innov. Food Sci. Emerg. Technol. 2009, 10, 356–362. [Google Scholar] [CrossRef]
- Giménez, M.; Olarte, C.; Sanz, S.; Lomas, C.; Echávarri, J.F.; Ayala, F. Relation between spoilage and microbiological quality in minimally processed artichoke packaged with different films. Food Microbiol. 2003, 20, 231–242. [Google Scholar] [CrossRef]
- Zhao, G.H.; Kapur, N.; Carlin, B.; Selinger, E.; Guthrie, J.T. Characterisation of the interactive properties of microcrystalline cellulose-carboxymethyl cellulose hydrogels. Int. J. Pharm. 2011, 415, 95–101. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int. J. Biol. Macromol. 2019, 137, 247–252. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Quintero Saavedra, J.I.; Chiralt, A. Antilisterial and physical properties of biopolymer films containing lactic acid bacteria. Food Control 2014, 35, 200–206. [Google Scholar] [CrossRef]
- Nadeem, H.Ş.; Koç, M.; Takma, D.K.; Duran, M. Recent Techniques for Packing and Storage of Spray-Dried Food Products. In Handbook on Spray Drying Applications for Food Industries; Selvamuthukumaran, M., Ed.; CRC Press: London, UK, 2019; pp. 271–306. [Google Scholar]
- Pandey, M.; Abeer, M.M.; Amin, M.C.I. Dissolution study of bacterial cellulose (nata de coco) from local food industry: Solubility behavior & structural changes. Int. J. Pharm. Pharm. Sci. 2014, 6, 89–93. [Google Scholar]
- Martins, D.; de Carvalho Ferreira, D.; Gama, M.; Dourado, F. Dry Bacterial Cellulose and Carboxymethyl Cellulose formulations with interfacial-active performance: Processing conditions and redispersion. Cellulose 2020, 27, 6505–6520. [Google Scholar] [CrossRef]
- Li, X.; Ren, Z.; Wang, R.; Liu, L.; Zhang, J.; Ma, F.; Khan, M.Z.H.; Zhao, D.; Liu, X. Characterization and antibacterial activity of edible films based on carboxymethyl cellulose, Dioscorea opposita mucilage, glycerol and ZnO nanoparticles. Food Chem. 2021, 349. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Vázquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT-Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Structural and physico-mechanical characterization of bio-cellulose produced by a cell-free system. Carbohydr. Polym. 2016, 136. [Google Scholar] [CrossRef]
- Costa, L.M.M.; de Olyveira, G.M.; Basmaji, P.; Lauro Filho, X. Bacterial cellulose towards functional green composites materials. J. Bionanoscience 2011, 5, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Casaburi, A.; Montoya Rojo, Ú.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl cellulose with tailored degree of substitution obtained from bacterial cellulose. Food Hydrocoll. 2018. [Google Scholar] [CrossRef]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty acid profile and biological activities of linseed and rapeseed oils. Molecules 2015, 20, 9887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, M.; Yuan, Z.; Qin, W.; Poirier, M.A.; Xu, C. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2015, 1, 1–7. [Google Scholar]
- Naumann, D. Infrared spectroscopy in microbiology. Encycl. Anal. Chem. Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Li, S.-M.; Jia, N.; Zhu, J.-F.; Ma, M.-G.; Sun, R.-C. Synthesis of cellulose–calcium silicate nanocomposites in ethanol/water mixed solvents and their characterization. Carbohydr. Polym. 2010, 80, 270–275. [Google Scholar] [CrossRef]
- Silviana, S.; Khusna, E.I.; Susanto, G.A.H.; Sanyoto, G.J.; Hadiyanto, H. Biocomposite of bacterial cellulose based from yam bean (Pachyrhizus erosus L. Urban) reinforced by bamboo microfibrillated cellulose through in situ method. AIP Conf. Proc. 2020, 2197, 50011. [Google Scholar]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Jang, J.H.; Park, J.K. In situ synthesis of a bio-cellulose/titanium dioxide nanocomposite by using a cell-free system. RSC Adv. 2016, 6, 22424–22435. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Zhang, M.; Ullah, M.W.; Liu, L.; Zhao, W.; Li, Y.; Ahmed, A.A.Q.; Cheng, H.; Shi, Z.; et al. In Situ Synthesized Selenium Nanoparticles-Decorated Bacterial Cellulose/Gelatin Hydrogel with Enhanced Antibacterial, Antioxidant, and Anti-Inflammatory Capabilities for Facilitating Skin Wound Healing. Adv. Healthc. Mater. 2021, 2100402. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Asselin, J.; Tazi, N.; Messaddeq, Y.; Levinson, D.; Zhang, Z. Production of Biocompatible and Antimicrobial Bacterial Cellulose Polymers Functionalized by RGDC Grafting Groups and Gentamicin. ACS Appl. Mater. Interfaces 2014, 6, 1439–1446. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018. [Google Scholar] [CrossRef]
- Kopp, J.; Slouka, C.; Ulonska, S.; Kager, J.; Fricke, J.; Spadiut, O.; Herwig, C. Impact of glycerol as carbon source onto specific sugar and inducer uptake rates and inclusion body productivity in E. coli BL21 (DE3). Bioengineering 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Mou, Y.; Shan, T.; Li, Y.; Zhou, L.; Wang, M.; Wang, J. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 2010, 15, 7961–7970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications: A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]











| Films | Moisture Content—WC (%) | Water Solubility—WS (%) |
|---|---|---|
| BC/CMC | 9.72 ± 0.32 | 22.28 ± 1.44 |
| BC/CMC/Gly | 30.11 ± 1.90 | 39.54 ± 2.30 |
| BC/CMC/Gly/yeast | 23.66 ± 1.59 | 42.86 ± 2.78 |
| Films | Tensile Strength (MPa) | Elongation at Break (%) a |
|---|---|---|
| BC | 17.02 ± 1.19 | 4.77 ± 0.56 |
| BC/CMC | 19.64 ± 1.43 | 4.61 ± 0.61 |
| BC/CMC/Gly | 5.01 ± 0.32 | 22.96 ± 1.24 |
| BC/CMC/Gly/yeast | 2.23 ± 0.33 | 15.53 ± 0.84 |
| Sample | Temperature (°C) | Oranges | Tomatoes | ||
|---|---|---|---|---|---|
| Minimum Accepted Value w.r.t Sensory Features | Minimum Accepted Value w.r.t Time (Days) | Minimum Accepted Value w.r.t Sensory Features | Minimum Accepted Value w.r.t Time (Days) | ||
| Control | 6 | 5 ± 0.35 | 49 | 5 ± 0.45 | 21 |
| 20–25 | 5 ± 0.4 | 14 | 5 ± 0.42 | 7 | |
| 30 | 6 ± 0.57 | 2 | 9 ± 0.81 | 2 | |
| 40 | 6 ± 0.54 | 2 | 8 ± 0.64 | 2 | |
| Film-0 | 6 | 5 ± 0.25 | 56 | 5 ± 0.5 | 28 |
| 20–25 | 5 ± 0.47 | 14 | 6 ± 0.54 | 7 | |
| 30 | 7 ± 0.77 | 2 | 9 ± 0.63 | 2 | |
| 40 | 6 ± 0.54 | 7 | 7 ± 0.66 | 2 | |
| Film-1 | 6 | 7 ± 0.52 | 63 | 7 ± 0.56 | 28 |
| 20–25 | 5 ± 0.25 | 28 | 5 ± 0.35 | 21 | |
| 30 | 6 ± 0.42 | 28 | 7 ± 0.56 | 14 | |
| 40 | 6 ± 0.48 | 21 | 7 ± 0.52 | 14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, O.M.; Manan, S.; Ahmed, A.A.Q.; Awad, M.F.; Ul-Islam, M.; Subhan, F.; Ullah, M.W.; Yang, G. Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application. Polymers 2021, 13, 2310. https://doi.org/10.3390/polym13142310
Atta OM, Manan S, Ahmed AAQ, Awad MF, Ul-Islam M, Subhan F, Ullah MW, Yang G. Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application. Polymers. 2021; 13(14):2310. https://doi.org/10.3390/polym13142310
Chicago/Turabian StyleAtta, Omar Mohammad, Sehrish Manan, Abeer Ahmed Qaed Ahmed, Mohamed F. Awad, Mazhar Ul-Islam, Fazli Subhan, Muhammad Wajid Ullah, and Guang Yang. 2021. "Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application" Polymers 13, no. 14: 2310. https://doi.org/10.3390/polym13142310
APA StyleAtta, O. M., Manan, S., Ahmed, A. A. Q., Awad, M. F., Ul-Islam, M., Subhan, F., Ullah, M. W., & Yang, G. (2021). Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application. Polymers, 13(14), 2310. https://doi.org/10.3390/polym13142310