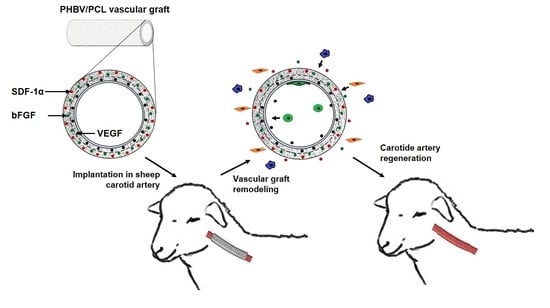
Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of TEVGs
2.2. Antithrombotic Modification of TEVG Luminal Surface
2.3. Evaluation of the Mechanical Properties
2.4. Haemocompatibility Testing
2.5. Visualisation of TEVG Structure
2.6. Animal Model
2.7. Histological, Immunofluorescent, and Ultrastructural Examination
2.8. Transcriptional Profiling
2.9. Statistical Analysis
3. Results
3.1. Hep/Ilo-Treated TEVGs Are Mechanically Competent
3.2. Conjugation of Hep/Ilo with the Luminal Surface of TEVGs Improves Their Haemocompatibility
3.3. Immobilisation of Hep/Ilo at the Luminal Surface of TEVGs by Poly(N-vinylpyrrolidone) Preserves Their Structure
3.4. Hep/Ilo Coating Endows TEVGs with a Considerable Long-Term Primary Patency
3.5. Cellular and Molecular Composition of PHBV/PCL[VEGF-bFGF-SDF]Hep/Ilo Grafts Is Reminiscent of Native Arteries
3.6. Biostable ePTFE Vascular Prostheses Undergo Thrombotic Occlusion and Calcification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2020 Update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Alqahtani, F.; Kalra, A.; Gafoor, S.; Alhajji, M.; Alreshidan, M.; Holmes, D.R.; Lerman, A. Trends in characteristics and outcomes of patients undergoing coronary revascularization in the United States, 2003–2016. JAMA Netw. Open 2020, 3, e1921326. [Google Scholar] [CrossRef]
- Blumenfeld, O.; Na’amnih, W.; Shapira-Daniels, A.; Lotan, C.; Shohat, T.; Shapira, O.M. Trends in coronary revascularization and ischemic heart disease-related mortality in Israel. J. Am. Heart Assoc. 2017, 6, e004734. [Google Scholar] [CrossRef] [Green Version]
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Saraiva, F.A.; Leite-Moreira, J.P.; Barros, A.S.; Lourenço, A.P.; Benedetto, U.; Leite-Moreira, A.F. Multiple versus single arterial grafting in coronary artery bypass grafting: A meta-analysis of randomized controlled trials and propensity score studies. Int. J. Cardiol. 2020, 320, 55–63. [Google Scholar] [CrossRef]
- Lejay, A.; Vento, V.; Kuntz, S.; Steinmetz, L.; Georg, Y.; Thaveau, F.; Heim, F.; Chakfé, N. Current status on vascular substitutes. J. Cardiovasc. Surg. 2020, 61, 55–63. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.W.; Cho, H.J.; Park, C.H.; Kim, C.S.; Jeong, I.S. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: A review. Cardiovasc. Eng. Technol. 2020, 11, 495–521. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The evolution of tissue engineered vascular graft technologies: From preclinical trials to advancing patient care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Wu, Z. Strategies in cell-free tissue-engineered vascular grafts. J. Biomed. Mater. Res. A 2020, 108, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Toong, D.W.Y.; Toh, H.W.; Ng, J.C.K.; Wong, P.E.H.; Leo, H.L.; Venkatraman, S.; Tan, L.P.; Ang, H.Y.; Huang, Y. Bioresorbable polymeric scaffold in cardiovascular applications. Int. J. Mol. Sci. 2020, 21, 3444. [Google Scholar] [CrossRef]
- Wang, Z.; Mithieux, S.M.; Weiss, A.S. Fabrication techniques for vascular and vascularized tissue engineering. Adv. Healthc. Mater. 2019, 8, e1900742. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, M.; Madeddu, P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front. Bioeng. Biotechnol. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue engineering at the blood-contacting surface: A review of challenges and strategies in vascular graft development. Adv. Healthc. Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, C.; Fioravanzo, L.; Folin, M.; Marchi, F.; Ercolani, E.; Bianco, A. Electrospun tubular scaffolds: On the effectiveness of blending poly(ε-caprolactone) with poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1883–1898. [Google Scholar] [CrossRef]
- Bye, F.J.; Bissoli, J.; Black, L.; Bullock, A.J.; Puwanun, S.; Moharamzadeh, K.; Reilly, G.C.; Ryan, A.J.; Neil, S.M. Development of bilayer and trilayer nanofibrous/microfibrous scaffolds for regenerative medicine. Biomater. Sci. 2013, 1, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Pařízek, M.; Novotná, K.; Bačáková, L. The role of smooth muscle cells in vessel wall pathophysiology and reconstruction using bioactive synthetic polymers. Physiol. Res. 2011, 60, 419–437. [Google Scholar] [CrossRef]
- Chan-Park, M.B.; Shen, J.Y.; Cao, Y.; Xiong, Y.; Liu, Y.; Rayatpisheh, S.; Kang, G.C.; Greisler, H.P. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J. Biomed. Mater. Res. A 2009, 88, 1104–1121. [Google Scholar] [CrossRef]
- Xie, S.A.; Zhang, T.; Wang, J.; Zhao, F.; Zhang, Y.P.; Yao, W.J.; Hur, S.S.; Yeh, Y.T.; Pang, W.; Zheng, L.S.; et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: Role of DNA methyltransferase 1. Biomaterials 2018, 155, 203–216. [Google Scholar] [CrossRef]
- Dayekh, K.; Mequanint, K. The effects of progenitor and differentiated cells on ectopic calcification of engineered vascular tissues. Acta Biomater. 2020, 115, 288–298. [Google Scholar] [CrossRef]
- Roh, J.D.; Sawh-Martinez, R.; Brennan, M.P.; Jay, S.M.; Devine, L.; Rao, D.A.; Yi, T.; Mirensky, T.L.; Nalbandian, A.; Udelsman, B.; et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 4669–4674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Gade, P.S.; Dong, L.; Zhang, Z.; Aral, A.M.; Gao, J.; Ding, X.; Stowell, C.E.T.; Nisar, M.U.; Kim, K.; et al. A biodegradable synthetic graft for small arteries matches the performance of autologous vein in rat carotid arteries. Biomaterials 2018, 181, 67–80. [Google Scholar] [CrossRef]
- Stowell, C.E.T.; Li, X.; Matsunaga, M.H.; Cockreham, C.B.; Kelly, K.M.; Cheetham, J.; Tzeng, E.; Wang, Y. Resorbable vascular grafts show rapid cellularization and degradation in the ovine carotid. J. Tissue Eng. Regen. Med. 2020, 14, 1673–1684. [Google Scholar] [CrossRef]
- Cai, Q.; Liao, W.; Xue, F.; Wang, X.; Zhou, W.; Li, Y.; Zeng, W. Selection of different endothelialization modes and different seed cells for tissue-engineered vascular graft. Bioact. Mater. 2021, 6, 2557–2568. [Google Scholar] [CrossRef]
- Sánchez, P.F.; Brey, E.M.; Briceño, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Krivkina, E.O.; Mironov, A.V.; Burago, A.Y.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; et al. Bioabsorbable bypass grafts biofunctionalised with RGD have enhanced biophysical properties and endothelialisation tested in vivo. Front. Pharmacol. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mironov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) small-diameter vascular grafts in vivo. Front. Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef] [Green Version]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD peptides and incorporation of vascular endothelial growth factor are equally efficient for biofunctionalization of tissue-engineered vascular grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of vascular graft studies in large animal models. Tissue Eng. Part B Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef]
- Swartz, D.D.; Andreadis, S.T. Animal models for vascular tissue-engineering. Curr. Opin. Biotechnol. 2013, 24, 916–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.V.; Lekshmi, V.; Nair, P.D. Tissue engineered vascular grafts--preclinical aspects. Int. J. Cardiol. 2013, 167, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Silnikov, V.N.; Sevostyanova, V.V.; Yuzhalin, A.E.; Koroleva, L.S.; Velikanova, E.A.; Mironov, A.V.; Godovikova, T.S.; Kutikhin, A.G.; Glushkova, T.V.; et al. Biocompatibility of small-diameter vascular grafts in different modes of RGD modification. Polymers 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Antonova, L.; Kutikhin, A.; Sevostianova, V.; Velikanova, E.; Matveeva, V.; Glushkova, T.; Mironov, A.; Krivkina, E.; Shabaev, A.; Senokosova, E.; et al. bFGF and SDF-1α improve in vivo performance of VEGF-incorporating small-diameter vascular grafts. Pharmaceuticals 2021, 14, 302. [Google Scholar] [CrossRef]
- Dimitrievska, S.; Niklason, L.E. Historical perspective and future direction of blood vessel developments. Cold Spring Harb. Perspect. Med. 2018, 8, a025742. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly(N-vinylpyrrolidone)-modified surfaces for biomedical applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, C.; Robins, S.P. Immunolocalization of collagen types I and III in the arterial wall of the rat. Histochem. J. 1989, 21, 172–178. [Google Scholar] [CrossRef] [PubMed]
- De Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Tara, S.; Kurobe, H.; Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Yi, T.; Naito, Y.; Breuer, C.K.; Shinoka, T. Well-organized neointima of large-pore poly(L-lactic acid) vascular graft coated with poly(L-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(L-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis 2014, 237, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kalinkevich, A.N.; Moskalenko, R.A.; Kuznetsov, V.N.; Kochenko, A.V.; Husak, E.V.; Starikov, V.V.; Liu, F.; Meng, J.; Lü, J. Structural and crystal-chemical characteristics of the apatite deposits from human aortic walls. Interv. Med. Appl. Sci. 2018, 10, 110–119. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [Green Version]
- Fukunishi, T.; Ong, C.S.; Yesantharao, P.; Best, C.A.; Yi, T.; Zhang, H.; Mattson, G.; Boktor, J.; Nelson, K.; Shinoka, T.; et al. Different degradation rates of nanofiber vascular grafts in small and large animal models. J. Tissue Eng. Regen. Med. 2020, 14, 203–214. [Google Scholar] [CrossRef]
- Tara, S.; Kurobe, H.; Maxfield, M.W.; Rocco, K.A.; Yi, T.; Naito, Y.; Breuer, C.K.; Shinoka, T. Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft. J. Vasc. Surg. 2015, 62, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, F.; Paefgen, V.; Winz, O.; Mertens, M.; Koch, S.; Gross-Weege, N.; Morgenroth, A.; Rix, A.; Schnoeringm, H.; Chalabi, K.; et al. MR and PET-CT monitoring of tissue-engineered vascular grafts in the ovine carotid artery. Biomaterials 2019, 216, 119228. [Google Scholar] [CrossRef]
- Cummings, I.; George, S.; Kelm, J.; Schmidt, D.; Emmert, M.Y.; Weber, B.; Zünd, G.; Hoerstrup, S.P. Tissue-engineered vascular graft remodeling in a growing lamb model: Expression of matrix metalloproteinases. Eur. J. Cardio Thorac. Surg. 2012, 41, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Shum-Tim, D.; Stock, U.; Hrkach, J.; Shinoka, T.; Lien, J.; Moses, M.A.; Stamp, A.; Taylor, G.; Moran, A.M.; Landis, W.; et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann. Thorac. Surg. 1999, 68, 2298–2304. [Google Scholar] [CrossRef]
- Naito, Y.; Williams-Fritze, M.; Duncan, D.R.; Church, S.N.; Hibino, N.; Madri, J.A.; Humphrey, J.D.; Shinoka, T.; Breuer, C.K. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs 2012, 195, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| IL1B | 5′-TGCTGAAGGCTCTCCACCTC-3′ | 5′-ACCCAAGGCCACAGGAATCTT-3′ |
| IL6 | 5′-TGTCATGGAGTTGCAGAGCAGT-3′ | 5′-CCAGCATGTCAGTGTGTGTGG-3′ |
| CXCL8 | 5′-CTTCCAAGCTGGCTGTTGCTC-3′ | 5′-ATTTGGGGTGGAAAGGTGTGG-3′ |
| ICAM1 | 5′-GTCACGGGGAACAGATTGTAGC-3′ | 5′-TGAGTTCTTCACCCACAGGCT-3′ |
| MMP2 | 5′-ACCCCGCTACGGTTTTCTCG-3′ | 5′-ATGAGCCAGGAGCCCGTCTT-3′ |
| KDR | 5′-ACAGAACCAAGTTAGCCCCATC-3′ | 5′-TCGCTGGAGTACACAGTGGTG-3′ |
| NR2F2 | 5′-GCAAGCGGTTTGGGACCTT-3′ | 5′-GGACAGGTAGGAGTGGCAGTTG-3′ |
| SNAI2 | 5′-ACCCTGGTTACTGCAAGGACA-3′ | 5′-GAGCCCTCAGATTGGACCTG-3′ |
| GAPDH | 5′-TGGTGAAGGTCGGAGTGAACG-3′ | 5′-AGGGGTCATTGATGGCAACG-3′ |
| B2M | 5′-CCTTCTGTCCCACGCTGAGT-3′ | 5′-TGGTGCTGCTTAGAGGTCTCG-3′ |
| PHBV/PCL [VEGF-bFGF-SDF] (n = 9) Median (IQR) | PHBV/PCL [VEGF-bFGF-SDF]Hep/Ilo (n = 9) Median (IQR) | ePTFE (n = 9) Median (IQR) | IMA (n = 9) Median (IQR) | |
|---|---|---|---|---|
| Ultimate Tensile Strength, MPa | 3.05 (2.90; 3.20) & | 3.94 (3.78–3.99) & | 22.95 (22.42–23.47) ** | 2.48 (1.36–3.25) & |
| Ultimate Tensile Force, N | 2.30 (2.20; 2.50) #,& | 3.08 (2.94–3.30) #,& | 21.10 (20.60–21.60) ** | 0.92 (0.59–1.72) & |
| Elongation at Break, % | 121.70 (117.1; 129.6) #,& | 109,17 (92.29–116.06) #,& | 337.00 (332.00–341.80) ** | 29.72 (23.51–39.62) & |
| Elastic Modulus, MPa | 8.60 (8.00; 9.64) #,& | 49.95 (44.90–54.70) *,#,& | 1.98 (1.36–2.59) | 2.42 (1.87–3.19) |
| Sample Thickness, mm | 0.36 (0.34–0.39) | 0.39 (0.39–0.40) | 0.46 (0.46–0.46) | 0.27 (0.24–0.30) |
| Graft Type | Haemolysis, % Median (IQR) | Maximum Platelet Aggregation, % Median (IQR) |
|---|---|---|
| Platelet-rich plasma | - | 14.61 (13.63–17.72) |
| PHBV/PCL[VEGF-bFGF-SDF] | 0 (0–0) | 17.25 (16.30–17.96) |
| PHBV/PCL[VEGF-bFGF-SDF]Hep/Ilo | 0.36 (0.36–0.36) ** | 8.22 (8.13–8.78) * |
| ePTFE | 0.33 (0.21–2.40) ** | 22.74 (22.45–24.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, L.V.; Krivkina, E.O.; Sevostianova, V.V.; Mironov, A.V.; Rezvova, M.A.; Shabaev, A.R.; Tkachenko, V.O.; Krutitskiy, S.S.; Khanova, M.Y.; Sergeeva, T.Y.; et al. Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model. Polymers 2021, 13, 2637. https://doi.org/10.3390/polym13162637
Antonova LV, Krivkina EO, Sevostianova VV, Mironov AV, Rezvova MA, Shabaev AR, Tkachenko VO, Krutitskiy SS, Khanova MY, Sergeeva TY, et al. Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model. Polymers. 2021; 13(16):2637. https://doi.org/10.3390/polym13162637
Chicago/Turabian StyleAntonova, Larisa V., Evgenia O. Krivkina, Viktoriia V. Sevostianova, Andrey V. Mironov, Maria A. Rezvova, Amin R. Shabaev, Vadim O. Tkachenko, Sergey S. Krutitskiy, Mariam Yu. Khanova, Tatiana Yu. Sergeeva, and et al. 2021. "Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model" Polymers 13, no. 16: 2637. https://doi.org/10.3390/polym13162637
APA StyleAntonova, L. V., Krivkina, E. O., Sevostianova, V. V., Mironov, A. V., Rezvova, M. A., Shabaev, A. R., Tkachenko, V. O., Krutitskiy, S. S., Khanova, M. Y., Sergeeva, T. Y., Matveeva, V. G., Glushkova, T. V., Kutikhin, A. G., Mukhamadiyarov, R. A., Deeva, N. S., Akentieva, T. N., Sinitsky, M. Y., Velikanova, E. A., & Barbarash, L. S. (2021). Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model. Polymers, 13(16), 2637. https://doi.org/10.3390/polym13162637