Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model
Abstract
:1. Introduction
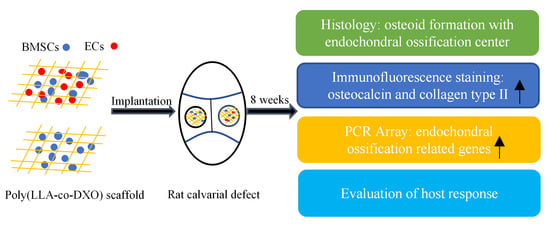
2. Materials and Methods
2.1. Preparation of Scaffolds
2.2. Cell Culturing, Cell Seeding
2.3. Graft Implantation
2.4. Histological Evaluation
2.5. Immunofluorescence Staining for Collagen Type II
2.6. Immunofluorescence Staining for Osteocalcin (OC)
2.7. RT2 Profiler PCR Array
2.8. Statistical Analysis
3. Results
3.1. Masson’s Trichrome Staining
3.2. Safranin O Staining
3.3. HE Staining
3.4. Expression of Collagen Type II
3.5. OC Expression
3.6. PCR Array Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.F.X.B.; Mazzon, E.; Trubiani, O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Int. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef] [Green Version]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Della Coletta, B.B.; Jacob, T.B.; Moreira, L.A.D.C.; Pomini, K.T.; Buchaim, D.V.; Eleutério, R.G.; Pereira, E.D.S.B.M.; Roque, D.D.; Rosso, M.P.D.O.; Shindo, J.V.T.C.; et al. Photobiomodulation Therapy on the Guided Bone Regeneration Process. in Defects Filled by Biphasic Calcium Phosphate Associated with Fibrin Biopolymer. Molecules 2021, 26, 847. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Tomin, E.; Bostrom, M.P.G. Biosynthetic Bone Grafting. Clin. Orthop. Relat. Res. 1999, 367, S107–S117. [Google Scholar] [CrossRef]
- Rosso, M.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.; Ferreira Júnior, R.S.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.B.; et al. Photobiomodulation Therapy Associated with Heterologous Fibrin Biopolymer and Bovine Bone Matrix Helps to Reconstruct Long Bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Huang, Y.; Ma, C.; Wu, C.; Tian, W. Current advances for bone regeneration based on tissue engineering strategies. Front. Med. 2019, 13, 160–188. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Corselli, M.; Petrigliano, F.A.; Soo, C.; Péault, B. Recent insights into the identity of mesenchymal stem cells: Implications for orthopaedic applications. Bone Joint J. 2014, 96, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhou, Z.; Ju, X.; Zhou, Y.; Lan, J.; Chen, N.; Chen, H.; Liu, M.; Pang, L. Combined transplantation of mesenchymal stem cells and endothelial progenitor cells for tissue engineering: A systematic review and meta-analysis. Stem Cell Res. Ther. 2016, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Cunniffe, G.M.; Dickson, G.R.; Partap, S.; Stanton, K.T.; O’Brien, F.J. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2293–2298. [Google Scholar] [CrossRef]
- Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized Scaffold in Bone Tissue Repair. Int. J. Mol. Sci. 2018, 19, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Meijer, G.J.; De Bruijn, J.D.; Koole, R.; Van Blitterswijk, C.A. Cell based bone tissue engineering in jaw defects. Biomaterials 2008, 29, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Steijn-Myagkaya, G.L. Death of osteocytes. Electron microscopy after in vitro ischaemia. J. Bone Joint Surg. Br. 1986, 68, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef] [Green Version]
- Thompson, E.M.; Matsiko, A.; Farrell, E.; Kelly, D.J.; O’Brien, F.J. Recapitulating endochondral ossification: A promising route to in vivo bone regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xing, Z.; Hellem, S.; Arvidson, K.; Mustafa, K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed. Eng. Online 2009, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Xue, Y.; Dånmark, S.; Schander, K.; Østvold, S.; Arvidson, K.; Hellem, S.; Finne-Wistrand, A.; Albertsson, A.-C.; Mustafa, K. Effect of endothelial cells on bone regeneration using poly(L-lactide-co-1,5-dioxepan-2-one) scaffolds. J. Biomed. Mater. Res. Part A 2010, 96, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.B.; Arvidson, K.; Plikk, P.; Ibrahim, S.; Finne-Wistrand, A.; Albertsson, A.-C.; Bolstad, A.I.; Mustafa, K. Polyester copolymer scaffolds enhance expression of bone markers in osteoblast-like cells. J. Biomed. Mater. Res. Part A 2010, 94, 631–639. [Google Scholar] [CrossRef]
- Xue, Y.; Dånmark, S.; Xing, Z.; Arvidson, K.; Albertsson, A.-C.; Hellem, S.; Finne-Wistrand, A.; Mustafa, K. Growth and differentiation of bone marrow stromal cells on biodegradable polymer scaffolds: An in vitro study. J. Biomed. Mater. Res. Part A 2010, 95, 1244–1251. [Google Scholar] [CrossRef]
- Finne-Wistrand, A.; Albertsson, A.-C.; Kwon, O.H.; Kawazoe, N.; Chen, G.; Kang, I.-K.; Hasuda, H.; Gong, J.; Ito, Y. Resorbable Scaffolds from Three Different Techniques: Electrospun Fabrics, Salt-Leaching Porous Films, and Smooth Flat Surfaces. Macromol. Biosci. 2008, 8, 951–959. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Yang, Z.; Han, B. Switch of macrophage fusion competency by 3D matrices. Sci. Rep. 2020, 10, 10348. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In vivo cellular reactions to different biomaterials—Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-Y.; Yu, H.; Gong, W.; Wu, B.; Mayton, L.; Costello, R.; Wooley, P.H. Murine model of prosthesis failure for the long-term study of aseptic loosening. J. Orthop. Res. 2007, 25, 603–611. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef]
- Dånmark, S.; Finne-Wistrand, A.; Wendel, M.; Arvidson, K.; Albertsson, A.-C.; Mustafa, K. Osteogenic Differentiation by Rat Bone Marrow Stromal Cells on Customized Biodegradable Polymer Scaffolds. J. Bioact. Compat. Polym. 2010, 25, 207–223. [Google Scholar] [CrossRef]
- Tusher, V.G.; Tibshirani, R.; Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 2001, 98, 5116–5121. [Google Scholar] [CrossRef] [Green Version]
- Zafeirakis, A. Collagenous and non-collagenous biochemical markers of bone metastases from prostate cancer. Hippokratia 2010, 14, 164–169. [Google Scholar]
- Boskey, A.; Gadaleta, S.; Gundberg, C.; Doty, S.; Ducy, P.; Karsenty, G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 1998, 23, 187–196. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nat. Cell Biol. 1996, 382, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Doherty, M.J.; Ashton, B.A.; Walsh, S.; Beresford, J.N.; Grant, M.E.; Canfield, A.E. Vascular Pericytes Express Osteogenic Potential In Vitro and In Vivo. J. Bone Miner. Res. 1998, 13, 828–838. [Google Scholar] [CrossRef]
- Gerstenfeld, L.; Landis, W. Gene-Expression and Extracellular-Matrix Ultrastructure of A Mineralizing Chondrocyte Cell-Culture System. J. Cell Biol. 1991, 112, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Yang, F.; Chu, W.; Zhao, H.; McMahon, C.; Li, C. Lentiviral-Mediated RNAi Knockdown of Cbfa1 Gene Inhibits Endochondral Ossification of Antler Stem Cells in Micromass Culture. PLoS ONE 2012, 7, e47367. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Mishina, Y.; Chen, D.; Huang, H.; Dallas, S.L.; Dallas, M.R.; Sivakumar, P.; Kunieda, T.; Tsutsui, T.W.; Boskey, A.; et al. Dmp1-deficient Mice Display Severe Defects in Cartilage Formation Responsible for a Chondrodysplasia-like Phenotype. J. Biol. Chem. 2005, 280, 6197–6203. [Google Scholar] [CrossRef] [Green Version]
- Sandell, L.J.; Nalin, A.M.; Reife, R.A. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev. Dyn. 1994, 199, 129–140. [Google Scholar] [CrossRef]
- Barbieri, O.; Astigiano, S.; Morini, M.; Tavella, S.; Schito, A.; Corsi, A.; Di Martino, D.; Bianco, P.; Cancedda, R.; Garofalo, S. Depletion of cartilage collagen fibrils in mice carrying a dominant negative Col2a1 transgene affects chondrocyte differentiation. Am. J. Cell Physiol. 2004, 285, C1504–C1512. [Google Scholar] [CrossRef] [Green Version]
- Deckers, M.M.L.; Van Beek, E.R.; Van Der Pluijm, G.; Wetterwald, A.; Van Der Wee-Pals, L.; Cecchini, M.G.; Papapoulos, S.E.; Löwik, C.W.G.M. Dissociation of Angiogenesis and Osteoclastogenesis During Endochondral Bone Formation in Neonatal Mice. J. Bone Miner. Res. 2002, 17, 998–1007. [Google Scholar] [CrossRef]
- Carlevaro, M.F.; Cermelli, S.; Cancedda, R.; Cancedda, F.D. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000, 113, 59–69. [Google Scholar] [CrossRef]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. 2001, 83 (Suppl. S2), S105–S115. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; De Bruijn, W.C. Foreign body reactions to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Bergsma, J.E.; De Bruijn, W.C.; Rozema, F.R.; Bos RR, M.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Böstman, O.; Hirvensalo, E.; Mäkinen, J.; Rokkanen, P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J. Bone Joint Surg. 1990, 72, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.H.; Sarmadi, M.; Helman, J.I.; Krebsbach, P.H. Effects of pH on human bone marrow stromal cells in vitro: Implications for tissue engineering of bone. J. Biomed. Mater. Res. 2002, 60, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Chakkalakal, D.A.; Mashoof, A.A.; Novak, J.; Strates, B.S.; McGuire, M.H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J. Biomed. Mater. Res. 1994, 28, 1439–1443. [Google Scholar] [CrossRef]
- Bartaula-Brevik, S.; Pedersen, T.O.; Blois, A.L.; Papadakou, P.; Finne-Wistrand, A.; Xue, Y.; Bolstad, A.I.; Mustafa, K. Leukocyte transmigration into tissue-engineered constructs is influenced by endothelial cells through Toll-like receptor signaling. Stem Cell Res. Ther. 2014, 5, 143. [Google Scholar] [CrossRef] [Green Version]
- Brodbeck, W.G.; Anderson, J.M. Giant cell formation and function. Curr. Opin. Hematol. 2009, 16, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Position | Gene | Full Name | Fold Change |
|---|---|---|---|
| A02 | Alpl | Alkaline phosphatase | 2.04 |
| A03 | Ambn | Ameloblastin | 2.51 |
| B09 | Col1a1 | Collagen type I, alpha 1 | 2.75 |
| B10 | Col1a2 | Collagen type I, alpha 2 | 2.15 |
| B11 | Col2a1 | Collagen type II, alpha 1 | 23.92 |
| C10 | Ctsk | Cathepsin K | 2.03 |
| C11 | Dmp1 | Dentin matrix acidic phosphoprotein 1 | 10.94 |
| C12 | Egf | Epidermal growth factor | 2.73 |
| E01 | Itga2 | Integrin alpha 2 | 2.04 |
| E06 | Mmp10 | Matrix metallopeptidase 10 | 2.43 |
| E09 | Mmp9 | Matrix metallopeptidase 9 | 2.68 |
| G10 | Vdr | Vitamin D receptor | 3.08 |
| A11 | Bmp6 | Bone morphogenetic protein 6 | −2.50 |
| D09 | Gdf10 | Growth differentiation factor 10 | −2.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Jiang, X.; Si, Q.; Finne-Wistrand, A.; Liu, B.; Xue, Y.; Mustafa, K. Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model. Polymers 2021, 13, 1521. https://doi.org/10.3390/polym13091521
Xing Z, Jiang X, Si Q, Finne-Wistrand A, Liu B, Xue Y, Mustafa K. Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model. Polymers. 2021; 13(9):1521. https://doi.org/10.3390/polym13091521
Chicago/Turabian StyleXing, Zhe, Xiaofeng Jiang, Qingzong Si, Anna Finne-Wistrand, Bin Liu, Ying Xue, and Kamal Mustafa. 2021. "Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model" Polymers 13, no. 9: 1521. https://doi.org/10.3390/polym13091521
APA StyleXing, Z., Jiang, X., Si, Q., Finne-Wistrand, A., Liu, B., Xue, Y., & Mustafa, K. (2021). Endochondral Ossification Induced by Cell Transplantation of Endothelial Cells and Bone Marrow Stromal Cells with Copolymer Scaffold Using a Rat Calvarial Defect Model. Polymers, 13(9), 1521. https://doi.org/10.3390/polym13091521